Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis
- PMID: 20826776
- PMCID: PMC2975170
- DOI: 10.1074/jbc.M110.109579
Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis
Erratum in
-
Stress effects on FosB and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis.J Biol Chem. 2018 Jun 29;293(26):10041. doi: 10.1074/jbc.AAC118.004299. J Biol Chem. 2018. PMID: 29959278 Free PMC article. No abstract available.
Abstract
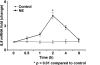
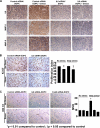
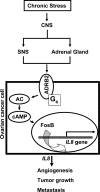
A growing number of studies indicate that chronic stress can accelerate tumor growth due to sustained sympathetic nervous system activation. Our recent findings suggest that chronic stress is associated with increased IL8 levels. Here, we examined the molecular and biological significance of IL8 in stress-induced tumor growth. Norepinephrine (NE) treatment of ovarian cancer cells resulted in a 250-300% increase in IL8 protein and 240-320% increase in its mRNA levels. Epinephrine treatment resulted in similar increases. Moreover, NE treatment resulted in a 3.5-4-fold increase in IL8 promoter activity. These effects were blocked by propranolol. Promoter deletion analyses suggested that AP1 transcription factors might mediate catecholamine-stimulated up-regulation of IL8. siRNA inhibition studies identified FosB as the pivotal component responsible for IL8 regulation by NE. In vivo chronic stress resulted in increased tumor growth (by 221 and 235%; p < 0.01) in orthotopic xenograft models involving SKOV3ip1 and HeyA8 ovarian carcinoma cells. This enhanced tumor growth was completely blocked by IL8 or FosB gene silencing using 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine nanoliposomes. IL8 and FosB silencing reduced microvessel density (based on CD31 staining) by 2.5- and 3.5-fold, respectively (p < 0.001). Our findings indicate that neurobehavioral stress leads to FosB-driven increases in IL8, which is associated with increased tumor growth and metastases. These findings may have implications for ovarian cancer management.
Figures






References
-
- Thaker P. H., Han L. Y., Kamat A. A., Arevalo J. M., Takahashi R., Lu C., Jennings N. B., Armaiz-Pena G., Bankson J. A., Ravoori M., Merritt W. M., Lin Y. G., Mangala L. S., Kim T. J., Coleman R. L., Landen C. N., Li Y., Felix E., Sanguino A. M., Newman R. A., Lloyd M., Gershenson D. M., Kundra V., Lopez-Berestein G., Lutgendorf S. K., Cole S. W., Sood A. K. (2006) Nat. Med. 12, 939–944 - PubMed
-
- Badino G. R., Novelli A., Girardi C., Di Carlo F. (1996) Pharmacol. Res. 33, 255–260 - PubMed
-
- Vandewalle B., Revillion F., Lefebvre J. (1990) J. Cancer Res. Clin. Oncol. 116, 303–306 - PubMed
-
- Marchetti B., Spinola P. G., Pelletier G., Labrie F. (1991) J. Steroid Biochem. Mol. Biol. 38, 307–320 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA101642/CA/NCI NIH HHS/United States
- HD050128/HD/NICHD NIH HHS/United States
- CA110793/CA/NCI NIH HHS/United States
- R01 CA104825/CA/NCI NIH HHS/United States
- R01 CA128797/CA/NCI NIH HHS/United States
- CA151668/CA/NCI NIH HHS/United States
- CA109298/CA/NCI NIH HHS/United States
- CA128797/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- RC2GM092599/GM/NIGMS NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- R01 CA110793/CA/NCI NIH HHS/United States
- RC2 GM092599/GM/NIGMS NIH HHS/United States
- T32 CA009614/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

