An interdomain interaction of the androgen receptor is required for its aggregation and toxicity in spinal and bulbar muscular atrophy
- PMID: 20826791
- PMCID: PMC2975181
- DOI: 10.1074/jbc.M110.146845
An interdomain interaction of the androgen receptor is required for its aggregation and toxicity in spinal and bulbar muscular atrophy
Abstract
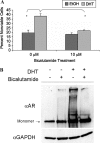
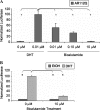
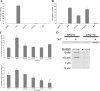
Polyglutamine expansion within the androgen receptor (AR) causes spinal and bulbar muscular atrophy (SBMA) and is associated with misfolded and aggregated species of the mutant AR. We showed previously that nuclear localization of the mutant AR was necessary but not sufficient for SBMA. Here we show that an interdomain interaction of the AR that is central to its function within the nucleus is required for AR aggregation and toxicity. Ligands that prevent the interaction between the amino-terminal FXXLF motif and carboxyl-terminal AF-2 domain (N/C interaction) prevented toxicity and AR aggregation in an SBMA cell model and rescued primary SBMA motor neurons from 5α-dihydrotestosterone-induced toxicity. Moreover, genetic mutation of the FXXLF motif prevented AR aggregation and 5α-dihydrotestosterone toxicity. Finally, selective androgen receptor modulators, which prevent the N/C interaction, ameliorated AR aggregation and toxicity while maintaining AR function, highlighting a novel therapeutic strategy to prevent the SBMA phenotype while retaining AR transcriptional function.
Figures







References
-
- Kennedy W. R., Alter M., Sung J. H. (1968) Neurology 18, 671–680 - PubMed
-
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. (1991) Nature 353, 77–79 - PubMed
-
- Pinsky L., Trifiro M., Kaufman M., Beitel L. K., Mhatre A., Kazemi-Esfarjani P., Sabbaghian N., Lumbroso R., Alvarado C., Vasiliou M., et al. (1992) Clin. Invest. Med. 15, 456–472 - PubMed
-
- Merry D. E., Kobayashi Y., Bailey C. K., Taye A. A., Fischbeck K. H. (1998) Hum. Mol. Genet. 7, 693–701 - PubMed
-
- Li M., Miwa S., Kobayashi Y., Merry D. E., Yamamoto M., Tanaka F., Doyu M., Hashizume Y., Fischbeck K. H., Sobue G. (1998) Ann. Neurol. 44, 249–254 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

