Sample size calculation in clinical trials: part 13 of a series on evaluation of scientific publications
- PMID: 20827353
- PMCID: PMC2933537
- DOI: 10.3238/arztebl.2010.0552
Sample size calculation in clinical trials: part 13 of a series on evaluation of scientific publications
Abstract
Background: In this article, we discuss the purpose of sample size calculation in clinical trials, the need for it, and the methods by which it is accomplished. Study samples that are either too small or too large are unacceptable, for clinical, methodological, and ethical reasons. The physicians participating in clinical trials should be directly involved in sample size planning, because their expertise and knowledge of the literature are indispensable.
Methods: We explain the process of sample size calculation on the basis of articles retrieved by a selective search of the international literature, as well as our own experience.
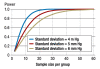
Results: We present a fictitious clinical trial in which two antihypertensive agents are to be compared to each other with a t-test and then show how the appropriate size of the study sample should be calculated. Next, we describe the general principles of sample size calculation that apply when any kind of statistical test is to be used. We give further illustrative examples and explain what types of expert medical knowledge and assumptions are needed to calculate the appropriate sample size for each. These generally depend on the particular statistical test that is to be performed.
Conclusion: In any clinical trial, the sample size has to be planned on a justifiable, rational basis. The purpose of sample size calculation is to determine the optimal number of participants (patients) to be included in the trial. Sample size calculation requires the collaboration of experienced biostatisticians and physician-researchers: expert medical knowledge is an essential part of it.
Figures
References
-
- Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227:309–313. - PubMed
-
- Halpern SD, Karlawish JHT, Berlin JA. The continuing unethical conduct of underpowered clinical trails. JAMA. 2002;288:358–362. - PubMed
-
- Altman DG. Practical Statistics for medical research. London: Chapman and Hall. 1991
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical