Therapeutic strategies for SLE involving cytokines: mechanism-oriented therapies especially IFN-gamma targeting gene therapy
- PMID: 20827419
- PMCID: PMC2933908
- DOI: 10.1155/2010/461641
Therapeutic strategies for SLE involving cytokines: mechanism-oriented therapies especially IFN-gamma targeting gene therapy
Abstract
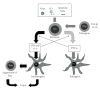
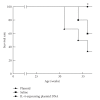
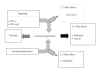
Systemic lupus erythematosus (SLE: lupus) is a chronic complicated autoimmune disease and pathogenesis is still unclear. However, key cytokines have been recognized. Interferon (IFN)-γ and also IFNalpha/beta are of particular importance. Depending on the concept that lupus is a helper T(Th)1 disease and that dendritic cells (DCs) determine the direction of lupus, balance shift of Th1/Th2 and immunogenic/tolerogenic DCs is reviewed for therapy. (IFN)-gamma- and IFN-alpha/beta-targeted (gene) therapies are introduced. These consist of Th1/Th2 balance shift and elimination of IFN-gamma and IFN-gamma-related cytokines such as (interleukin)IL-12 and IL-18. Other approaches include suppression of immunocompetent cells, normalization of abnormal T-cell function, costimulation blockade, B lymphocyte stimulator (Blys) blockade, and suppression of nephritic kidney inflammation. Moreover, balance shift of IFN-alpha/beta and tumor necrosis factor (TNF)-alpha together with regulatory T(Treg) cells are briefly introduced. Clinical application will be discussed.
Figures


Similar articles
-
Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity.Cytokine. 2015 Apr;72(2):146-53. doi: 10.1016/j.cyto.2014.12.027. Epub 2015 Jan 31. Cytokine. 2015. PMID: 25647269
-
Th1/Th2 cytokines in patients with systemic lupus erythematosus: is tumor necrosis factor alpha protective?Semin Arthritis Rheum. 2004 Jun;33(6):404-13. doi: 10.1016/j.semarthrit.2003.11.002. Semin Arthritis Rheum. 2004. PMID: 15190525
-
Elevated gene expression of Th1/Th2 associated transcription factors is correlated with disease activity in patients with systemic lupus erythematosus.J Rheumatol. 2007 Jan;34(1):89-96. Epub 2006 Nov 15. J Rheumatol. 2007. PMID: 17117487
-
Anti-interferon alpha treatment in SLE.Clin Immunol. 2013 Sep;148(3):303-12. doi: 10.1016/j.clim.2013.02.013. Epub 2013 Mar 1. Clin Immunol. 2013. PMID: 23566912 Review.
-
Stability of Regulatory T Cells Undermined or Endorsed by Different Type-1 Cytokines.Adv Exp Med Biol. 2015;850:17-30. doi: 10.1007/978-3-319-15774-0_2. Adv Exp Med Biol. 2015. PMID: 26324343 Review.
Cited by
-
Immunomodulation Mechanism of Antidepressants: Interactions between Serotonin/Norepinephrine Balance and Th1/Th2 Balance.Curr Neuropharmacol. 2012 Jun;10(2):97-123. doi: 10.2174/157015912800604542. Curr Neuropharmacol. 2012. PMID: 23204981 Free PMC article.
-
Altered AKT1 and MAPK1 gene expression on peripheral blood mononuclear cells and correlation with T-helper-transcription factors in systemic lupus erythematosus patients.Mediators Inflamm. 2012;2012:495934. doi: 10.1155/2012/495934. Epub 2012 Oct 18. Mediators Inflamm. 2012. PMID: 23125486 Free PMC article.
-
The Modulatory Roles of N-glycans in T-Cell-Mediated Autoimmune Diseases.Int J Mol Sci. 2018 Mar 8;19(3):780. doi: 10.3390/ijms19030780. Int J Mol Sci. 2018. PMID: 29518037 Free PMC article. Review.
-
Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases.Int J Mol Sci. 2013 May 31;14(6):11742-66. doi: 10.3390/ijms140611742. Int J Mol Sci. 2013. PMID: 23727938 Free PMC article. Review.
-
Therapeutic effect of Withania somnifera on pristane-induced model of SLE.Inflammopharmacology. 2012 Aug;20(4):195-205. doi: 10.1007/s10787-011-0102-8. Epub 2011 Dec 13. Inflammopharmacology. 2012. PMID: 22160928
References
-
- Klinman DM, Steinberg AD. Inquiry into murine and human lupus. Immunological Reviews. 1995;(144):157–193. - PubMed
-
- Koffler D. Immunopathogenesis of systemic lupus erythematosus. Annual Review of Medicine. 1974;25:149–164. - PubMed
-
- Doria A, Briani C. Lupus: improving long-term prognosis. Lupus. 2008;17(3):166–170. - PubMed
-
- Van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? European Journal of Clinical Investigation. 2009;39(2):81–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
