The Calcium-Dependent Interaction of S100B with Its Protein Targets
- PMID: 20827422
- PMCID: PMC2933916
- DOI: 10.1155/2010/728052
The Calcium-Dependent Interaction of S100B with Its Protein Targets
Abstract
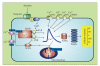
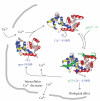
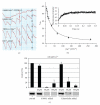
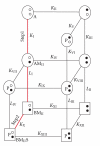
S100B is a calcium signaling protein that is a member of the S100 protein family. An important feature of S100B and most other S100 proteins (S100s) is that they often bind Ca(2+) ions relatively weakly in the absence of a protein target; upon binding their target proteins, Ca(2+)-binding then increases by as much as from 200- to 400-fold. This manuscript reviews the structural basis and physiological significance of increased Ca(2+)-binding affinity in the presence of protein targets. New information regarding redundancy among family members and the structural domains that mediate the interaction of S100B, and other S100s, with their targets is also presented. It is the diversity among individual S100s, the protein targets that they interact with, and the Ca(2+) dependency of these protein-protein interactions that allow S100s to transduce changes in [Ca(2+)](intracellular) levels into spatially and temporally unique biological responses.
Figures

Similar articles
-
Molecular mechanisms of S100-target protein interactions.Microsc Res Tech. 2003 Apr 15;60(6):552-9. doi: 10.1002/jemt.10297. Microsc Res Tech. 2003. PMID: 12645003 Review.
-
Ions binding to S100 proteins. I. Calcium- and zinc-binding properties of bovine brain S100 alpha alpha, S100a (alpha beta), and S100b (beta beta) protein: Zn2+ regulates Ca2+ binding on S100b protein.J Biol Chem. 1986 Jun 25;261(18):8192-203. J Biol Chem. 1986. PMID: 3722149
-
Solution structure of zinc- and calcium-bound rat S100B as determined by nuclear magnetic resonance spectroscopy.Biochemistry. 2005 Apr 19;44(15):5690-702. doi: 10.1021/bi0475830. Biochemistry. 2005. PMID: 15823027
-
The Zn2+ and Ca2+ -binding S100B and S100A1 proteins: beyond the myths.Biol Rev Camb Philos Soc. 2020 Jun;95(3):738-758. doi: 10.1111/brv.12585. Epub 2020 Feb 6. Biol Rev Camb Philos Soc. 2020. PMID: 32027773 Review.
-
Calcium-binding properties of wild-type and EF-hand mutants of S100B in the presence and absence of a peptide derived from the C-terminal negative regulatory domain of p53.Biochemistry. 2005 May 17;44(19):7305-14. doi: 10.1021/bi050321t. Biochemistry. 2005. PMID: 15882069
Cited by
-
S100A4 inhibits cell proliferation by interfering with the S100A1-RAGE V domain.PLoS One. 2019 Feb 19;14(2):e0212299. doi: 10.1371/journal.pone.0212299. eCollection 2019. PLoS One. 2019. PMID: 30779808 Free PMC article.
-
Critical role and its underlying molecular events of the plasminogen receptor, S100A10 in malignant tumor and non-tumor diseases.J Cancer. 2020 Jan 1;11(4):826-836. doi: 10.7150/jca.36203. eCollection 2020. J Cancer. 2020. PMID: 31949486 Free PMC article. Review.
-
Multiple Evolutionary Origins of Ubiquitous Cu2+ and Zn2+ Binding in the S100 Protein Family.PLoS One. 2016 Oct 20;11(10):e0164740. doi: 10.1371/journal.pone.0164740. eCollection 2016. PLoS One. 2016. PMID: 27764152 Free PMC article.
-
Transcriptomics of circulating neutrophils in dairy cows with subclinical hypocalcemia.Front Vet Sci. 2022 Sep 13;9:959831. doi: 10.3389/fvets.2022.959831. eCollection 2022. Front Vet Sci. 2022. PMID: 36176696 Free PMC article.
-
Functions of S100 proteins.Curr Mol Med. 2013 Jan;13(1):24-57. Curr Mol Med. 2013. PMID: 22834835 Free PMC article. Review.
References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology. 2003;4(7):517–529. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochemical Society symposium. 2007;(74):1–7. - PubMed
-
- Moore BW. A soluble protein characteristic of the nervous system. Biochemical and Biophysical Research Communications. 1965;19(6):739–744. - PubMed
-
- Ravasi T, Hsu K, Goyette J, et al. Probing the S100 protein family through genomic and functional analysis. Genomics. 2004;84(1):10–22. - PubMed
-
- Donato R. Intracellular and extracellular roles of S100 proteins. Microscopy Research and Technique. 2003;60(6):540–551. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
