The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit
- PMID: 20827424
- PMCID: PMC2933925
- DOI: 10.1177/1947601910373795
The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit
Abstract
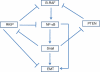
Melanoma is a highly metastatic cancer, and there are no current therapeutic modalities to treat this deadly malignant disease once it has metastasized. Melanoma cancers exhibit B-RAF mutations in up to 70% of cases. B-RAF mutations are responsible, in large part, for the constitutive hyperactivation of survival/antiapoptotic pathways such as the MAPK, NF-κB, and PI3K/AKT. These hyperactivated pathways regulate the expression of genes targeting the initiation of the metastatic cascade, namely, the epithelial to mesenchymal transition (EMT). EMT is the result of the expression of mesenchymal gene products such as fibronectin, vimentin, and metalloproteinases and the invasion and inhibition of E-cadherin. The above pathways cross-talk and regulate each other's activities and functions. For instance, the NF-κB pathway directly regulates EMT through the transcription of gene products involved in EMT and indirectly through the transcriptional up-regulation of the metastasis inducer Snail. Snail, in turn, suppresses the expression of the metastasis suppressor gene product Raf kinase inhibitor protein RKIP (inhibits the MAPK and the NF-κB pathways) as well as PTEN (inhibits the PI3K/AKT pathway). The role of B-RAF mutations in melanoma and their direct role in the induction of EMT are not clear. This review discusses the hypothesis that B-RAF mutations are involved in the dysregulation of the NF-κB/Snail/RKIP/PTEN circuit and in both the induction of EMT and metastasis. The therapeutic implications of the dysregulation of the above circuit by B-RAF mutations are such that they offer novel targets for therapeutic interventions in the treatment of EMT and metastasis.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



References
-
- Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta 2009;1796(2):293-308 - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59(4):225-49 - PubMed
-
- Grin JM, Grant-Kels JM, Grin CM, Berke A, Kels BD. Ocular melanomas and melanocytic lesions of the eye. J Am Acad Dermatol 1998;38(5 Pt 1):716-30 - PubMed
-
- Hussein MR. Extracutaneous malignant melanomas. Cancer Invest 2008;26(5):516-34 - PubMed
-
- Moan J, Porojnicu AC, Dahlback A. Ultraviolet radiation and malignant melanoma. Adv Exp Med Biol 2008;624:104-16 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
