Murine model of chronic L. (Viannia) panamensis infection: role of IL-13 in disease
- PMID: 20827674
- PMCID: PMC3289133
- DOI: 10.1002/eji.201040384
Murine model of chronic L. (Viannia) panamensis infection: role of IL-13 in disease
Abstract
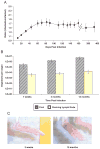
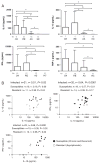
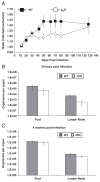
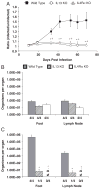
Leishmania (Viannia) organisms are the most prevalent etiologic agents of human cutaneous leishmaniasis in the Americas. Nevertheless, our knowledge of the immunological mechanisms exploited by L. (Viannia) organisms remains limited and the mechanisms underlying disease are not well understood. Here, we report the development of a BALB/c mouse model of L. (V.) panamensis infection that is able to reproduce chronic disease, with persistent infection and clinically evident lesions for over 1 year. The immune response of the mouse resembles that found for L. (V.) panamensis-infected patients with chronic and recurrent lesions, presenting a mixed Th1/Th2 response with the presence of TNF-α, IFN-γ, IL-10 and IL-13. Using immunodeficient mice, the critical role for IL-13 and/or IL-4Rα in determining susceptibility to chronic infection was evident. With the induction of healing in the immunodeficient mice, increases in IFN-γ and IL-17 were found, concomitant with parasite control and elimination. Specifically, increases in CD4(+) (but not CD8(+)) T cells producing IFN-γ were observed. These results suggest that IL-13 represents an important target for disease control of L. (V.) panamensis infection. This murine model should be useful to further understand the pathology associated with chronic disease and to develop methods for the treatment and prevention of leishmaniasis caused by L. (Viannia) parasites.
Conflict of interest statement
Figures
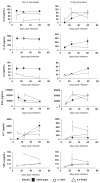

References
-
- WHO. Magnitude of the Problem. 2009 http://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/in....
-
- Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. - PubMed
-
- Bosque F, Saravia NG, Valderrama L, Milon G. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand J Immunol. 2000;51:533–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

