Recombinant human sperm-specific glyceraldehyde-3-phosphate dehydrogenase (GAPDHS) is expressed at high yield as an active homotetramer in baculovirus-infected insect cells
- PMID: 20828617
- PMCID: PMC2992962
- DOI: 10.1016/j.pep.2010.09.003
Recombinant human sperm-specific glyceraldehyde-3-phosphate dehydrogenase (GAPDHS) is expressed at high yield as an active homotetramer in baculovirus-infected insect cells
Erratum in
- Protein Expr Purif. 2011 Apr;76(2):264
Abstract
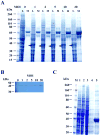
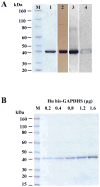
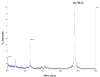
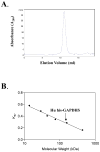
The sperm-specific glyceraldehyde-3-phosphate dehydrogenase (GAPDHS) isoform is a promising contraceptive target because it is specific to male germ cells, essential for sperm motility and male fertility, and well suited to pharmacological inhibition. However, GAPDHS is difficult to isolate from native sources and recombinant expression frequently results in high production of insoluble enzyme. We chose to use the Bac-to-Bac baculovirus-insect cell system to express a His-tagged form of human GAPDHS (Hu his-GAPDHS) lacking the proline-rich N-terminal sequence. This recombinant Hu his-GAPDHS was successfully produced in Spodoptera frugiperda 9 (Sf9) cells by infection with recombinant virus as a soluble, enzymatically active form in high yield, >35 mg/L culture. Biochemical characterization of the purified enzyme by mass spectrometry and size exclusion chromatography confirmed the presence of the tetrameric form. Further characterization by peptide ion matching mass spectrometry and Edman sequencing showed that unlike the mixed tetramer forms produced in bacterial expression systems, human his-GAPDHS expressed in baculovirus-infected insect cells is homotetrameric. The ability to express and purify active human GAPDHS as homotetramers in high amounts will greatly aid in drug discovery efforts targeting this enzyme for discovery of novel contraceptives and three compounds were identified as inhibitors of Hu his-GAPDHS from a pilot screen of 1120 FDA-approved compounds.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Welch J, Brown P, O’Brien D, Magyar P, Bunch D, Mori C, Eddy E. Human glyceraldehyde 3-phosphate dehydrogenase-2 gene is expressed specifically in spermatogenic cells. J Androl. 2000;21:328–338. - PubMed
-
- Welch J, Schatte E, O’Brien D, Eddy E. Expression of a glyceraldehyde 3-phosphate dehydrogenase gene specific to mouse spermatogenic cells. Biol Reprod. 1992;46:869–878. - PubMed
-
- Welch J, Barbee R, Magyar P, Bunch D, OBrien D. Expression of the spermatogenic cell-specific glyceraldehyde 3-phosphate dehydrogenase (GAPDS) in rat testis. Mol Reprod Devel. 2006;73:1052–1060. - PubMed
-
- Danshina P, Temple B, Williams K, Sexton J, Yeh L, O’Brien D. New strategies for male fertility control: sperm glycolytic enzymes as targets. J Androl. 2009;30:23.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

