Endothelial microparticles induce inflammation in acute lung injury
- PMID: 20828748
- PMCID: PMC4731030
- DOI: 10.1016/j.jss.2010.05.036
Endothelial microparticles induce inflammation in acute lung injury
Abstract
Background: Previously, we have shown that endothelial microparticles (EMPs) injected into mice induce acute lung injury (ALI) [1]. In this study, we hypothesize that EMPs induce ALI by initiating cytokine release in the lung, leading to recruitment and activation of neutrophils.
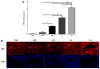
Materials and methods: C57BL/6J male mice (8-10 wk old) were intravenously injected with EMPs (200,000/mL), LPS (2 mg/kg), or both. Bronchoalveolar lavage (BAL) and serum levels of IL-1β and TNF-α were analyzed by enzyme-linked immunoassay (ELISA). Morphometric analysis was performed on H and E stained lung sections. Myeloperoxidase (MPO) levels were determined via an enzymatic assay and immunofluorescence of stained sections.
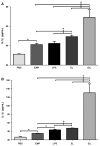
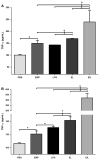
Results: EMPs led to significantly increased pulmonary and systemic IL-1β and TNF-α levels, which correlated with increased neutrophil recruitment to the lung. MPO levels in the lungs were increased significantly following injection of EMPs or LPS, compared to PBS. In mice treated with EMPs and LPS either simultaneously or successively, the cytokine and MPO levels were significantly increased over that of either treatment alone.
Conclusion: EMPs contribute to lung injury through the initiation of a cytokine cascade that increases recruitment of neutrophils and subsequent release of MPO. Furthermore, treatment of mice with both EMPs and LPS induced greater lung injury than either treatment alone, suggesting that EMPs prime the lung for increased injury by other pathogens. Therapies aimed at reducing or blocking EMPs may be a useful strategy for attenuating lung injury.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Densmore JC, Signorino PR, Ou J, et al. Endothelium-derived microparticles in duce endothelial dysfunction and acute lung injury. Shock. 2006;26:464. - PubMed
-
- Ogura H, Tanaka H, Koh T, et al. Enhanced production of endothelial microparticles with increased binding to leukocytes in patients with severe systemic inflammatory response syndrome. J Trauma. 2004;56:823. - PubMed
-
- Kang P, Shen B, Yang J, Pei F. Circulating platelet-derived microparticles and endothelium-derived microparticles may be a potential cause of microthrombosis in patients with osteonecrosis of the femoral head. Thromb Res. 2008;123:367. - PubMed
-
- Guiducci S, Distler JHW, Jüngel A, et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum. 2008;58:2845. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

