Glutaredoxin 2 prevents aggregation of mutant SOD1 in mitochondria and abolishes its toxicity
- PMID: 20829229
- PMCID: PMC3298854
- DOI: 10.1093/hmg/ddq383
Glutaredoxin 2 prevents aggregation of mutant SOD1 in mitochondria and abolishes its toxicity
Abstract
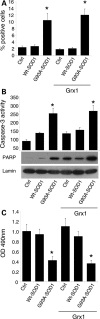
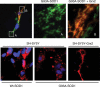
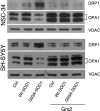
Vulnerability of motoneurons in amyotrophic lateral sclerosis (ALS) arises from a combination of several mechanisms, including protein misfolding and aggregation, mitochondrial dysfunction and oxidative damage. Protein aggregates are found in motoneurons in models for ALS linked to a mutation in the gene coding for Cu,Zn superoxide dismutase (SOD1) and in ALS patients as well. Aggregation of mutant SOD1 in the cytoplasm and/or into mitochondria has been repeatedly proposed as a main culprit for the degeneration of motoneurons. It is, however, still debated whether SOD1 aggregates represent a cause, a correlate or a consequence of processes leading to cell death. We have exploited the ability of glutaredoxins (Grxs) to reduce mixed disulfides to protein thiols either in the cytoplasm and in the IMS (Grx1) or in the mitochondrial matrix (Grx2) as a tool for restoring a correct redox environment and preventing the aggregation of mutant SOD1. Here we show that the overexpression of Grx1 increases the solubility of mutant SOD1 in the cytosol but does not inhibit mitochondrial damage and apoptosis induced by mutant SOD1 in neuronal cells (SH-SY5Y) or in immortalized motoneurons (NSC-34). Conversely, the overexpression of Grx2 increases the solubility of mutant SOD1 in mitochondria, interferes with mitochondrial fragmentation by modifying the expression pattern of proteins involved in mitochondrial dynamics, preserves mitochondrial function and strongly protects neuronal cells from apoptosis. The toxicity of mutant SOD1, therefore, mostly arises from mitochondrial dysfunction and rescue of mitochondrial damage may represent a promising therapeutic strategy.
Figures







Similar articles
-
Mutant SOD1-induced neuronal toxicity is mediated by increased mitochondrial superoxide levels.J Neurochem. 2007 Aug;102(3):609-18. doi: 10.1111/j.1471-4159.2007.04502.x. Epub 2007 Mar 23. J Neurochem. 2007. PMID: 17394531
-
Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities.Hum Mol Genet. 2009 Dec 1;18(23):4552-64. doi: 10.1093/hmg/ddp421. Epub 2009 Sep 24. Hum Mol Genet. 2009. PMID: 19779023 Free PMC article.
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
The human G93A-superoxide dismutase-1 mutation, mitochondrial glutathione and apoptotic cell death.Neurochem Res. 2009 Oct;34(10):1847-56. doi: 10.1007/s11064-009-9974-z. Epub 2009 Apr 28. Neurochem Res. 2009. PMID: 19399611 Review.
-
Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS.Prog Neurobiol. 2008 May;85(1):94-134. doi: 10.1016/j.pneurobio.2008.01.001. Epub 2008 Jan 16. Prog Neurobiol. 2008. PMID: 18282652 Review.
Cited by
-
Mechanisms of mutant SOD1 induced mitochondrial toxicity in amyotrophic lateral sclerosis.Front Cell Neurosci. 2014 May 9;8:126. doi: 10.3389/fncel.2014.00126. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24847211 Free PMC article. Review.
-
Redox regulation of mitochondrial function.Antioxid Redox Signal. 2012 Jun 1;16(11):1323-67. doi: 10.1089/ars.2011.4123. Epub 2012 Feb 3. Antioxid Redox Signal. 2012. PMID: 22146081 Free PMC article. Review.
-
Mitochondria Dysfunction in Frontotemporal Dementia/Amyotrophic Lateral Sclerosis: Lessons From Drosophila Models.Front Neurosci. 2021 Nov 24;15:786076. doi: 10.3389/fnins.2021.786076. eCollection 2021. Front Neurosci. 2021. PMID: 34899176 Free PMC article. Review.
-
Lithium facilitates removal of misfolded proteins and attenuated faulty interaction between mutant SOD1 and p-CREB (Ser133) through enhanced autophagy in mutant hSOD1G93A transfected neuronal cell lines.Mol Biol Rep. 2019 Dec;46(6):6299-6309. doi: 10.1007/s11033-019-05071-4. Epub 2019 Sep 16. Mol Biol Rep. 2019. PMID: 31529340
-
Non-native soluble oligomers of Cu/Zn superoxide dismutase (SOD1) contain a conformational epitope linked to cytotoxicity in amyotrophic lateral sclerosis (ALS).Biochemistry. 2014 Apr 15;53(14):2423-32. doi: 10.1021/bi500158w. Epub 2014 Apr 2. Biochemistry. 2014. PMID: 24660965 Free PMC article.
References
-
- Cozzolino M., Ferri A., Carri M.T. Amyotrophic lateral sclerosis: from current developments in the laboratory to clinical implications. Antioxid. Redox Signal. 2008;10:405–443. doi:10.1089/ars.2007.1760. - DOI - PubMed
-
- Chattopadhyay M., Valentine J.S. Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS. Antioxid. Redox Signal. 2009;11:1603–1614. doi:10.1089/ars.2009.2536. - DOI - PMC - PubMed
-
- Ferri A., Cozzolino M., Crosio C., Nencini M., Casciati A., Gralla E.B., Rotilio G., Valentine J.S., Carri M.T. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc. Natl Acad. Sci. USA. 2006;103:13860–13865. doi:10.1073/pnas.0605814103. - DOI - PMC - PubMed
-
- Cozzolino M., Pesaresi M.G., Amori I., Crosio C., Ferri A., Nencini M., Carri M.T. Oligomerization of mutant SOD1 in mitochondria of motoneuronal cells drives mitochondrial damage and cell toxicity. Antioxid. Redox Signal. 2009;11:1547–1558. doi:10.1089/ars.2009.2545. - DOI - PubMed
-
- Hurd T.R., Filipovska A., Costa N.J., Dahm C.C., Murphy M.P. Disulphide formation on mitochondrial protein thiols. Biochem. Soc. Trans. 2005;33:1390–1393. doi:10.1042/BST20051390. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

