Structural basis for the cAMP-dependent gating in the human HCN4 channel
- PMID: 20829353
- PMCID: PMC2978636
- DOI: 10.1074/jbc.M110.152033
Structural basis for the cAMP-dependent gating in the human HCN4 channel
Abstract
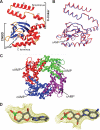
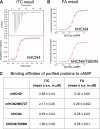
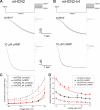
Hyperpolarization-activated cAMP-regulated (HCN) channels play important physiological roles in both cardiovascular and central nervous systems. Among the four HCN isoforms, HCN2 and HCN4 show high expression levels in the human heart, with HCN4 being the major cardiac isoform. The previously published crystal structure of the mouse HCN2 (mHCN2) C-terminal fragment, including the C-linker and the cyclic-nucleotide binding domain (CNBD), has provided many insights into cAMP-dependent gating in HCN channels. However, structures of other mammalian HCN channel isoforms have been lacking. Here we used a combination of approaches including structural biology, biochemistry, and electrophysiology to study cAMP-dependent gating in HCN4 channel. First we solved the crystal structure of the C-terminal fragment of human HCN4 (hHCN4) channel at 2.4 Å. Overall we observed a high similarity between mHCN2 and hHCN4 crystal structures. Functional comparison between two isoforms revealed that compared with mHCN2, the hHCN4 protein exhibited marked different contributions to channel function, such as a ∼3-fold reduction in the response to cAMP. Guided by structural differences in the loop region between β4 and β5 strands, we identified residues that could partially account for the differences in response to cAMP between mHCN2 and hHCN4 proteins. Moreover, upon cAMP binding, the hHCN4 C-terminal protein exerts a much prolonged effect in channel deactivation that could have significant physiological contributions.
Figures




References
-
- Hille B. (2001) Ion Channels of Excitable Membranes, 3rd Ed., Sinauer, Sunderland, MA
-
- Jan L. Y., Jan Y. N. (1990) Nature 345, 672. - PubMed
-
- Zagotta W. N., Siegelbaum S. A. (1996) Annu. Rev. Neurosci. 19, 235–263 - PubMed
-
- Biel M., Wahl-Schott C., Michalakis S., Zong X. (2009) Physiol. Rev. 89, 847–885 - PubMed
-
- Ganetzky B., Robertson G. A., Wilson G. F., Trudeau M. C., Titus S. A. (1999) Ann. N.Y. Acad. Sci. 868, 356–369 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

