Exploring challenges in rational enzyme design by simulating the catalysis in artificial kemp eliminase
- PMID: 20829491
- PMCID: PMC2947920
- DOI: 10.1073/pnas.1010381107
Exploring challenges in rational enzyme design by simulating the catalysis in artificial kemp eliminase
Abstract
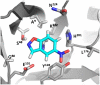
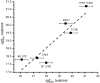
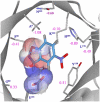
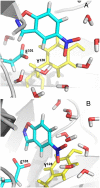
One of the fundamental challenges in biotechnology and in biochemistry is the ability to design effective enzymes. Doing so would be a convincing manifestation of a full understanding of the origin of enzyme catalysis. Despite an impressive progress, most of the advances on this front have been made by placing the reacting fragments in the proper places, rather than by optimizing the environment preorganization, which is the key factor in enzyme catalysis. Rational improvement of the preorganization would require approaches capable of evaluating reliably the actual catalytic effect. This work takes previously designed kemp eliminases as a benchmark for a computer aided enzyme design, using the empirical valence bond as the main screening tool. The observed absolute catalytic effect and the effect of directed evolution are reproduced and analyzed (assuming that the substrate is in the designed site). It is found that, in the case of kemp eliminases, the transition state charge distribution makes it hard to exploit the active site polarity, even with the ability to quantify the effect of different mutations. Unexpectedly, it is found that the directed evolution mutants lead to the reduction of solvation of the reactant state by water molecules rather that to the more common mode of transition state stabilization used by naturally evolved enzymes. Finally it is pointed out that our difficulties in improving Kemp eliminase are not due to overlooking exotic effect, but to the challenge in designing a preorganized environment that would exploit the small change it charge distribution during the formation of the transition state.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
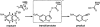

References
-
- Toscano MD, Woycechowsky KJ, Hilvert D. Minimalist active-site redesign: Teaching old enzymes new tricks. Angew Chem Int Edit. 2007;46:4468–4470. - PubMed
-
- Khersonsky O, et al. Evolutionary optimization of computationally designed enzymes: Kemp eliminases of the KE07 series. J Mol Biol. 2010;396:1025–1042. - PubMed
-
- Warshel A, et al. Electrostatic basis for enzyme catalysis. Chem Rev. 2006;106:3210–3235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

