Functional roles for noise in genetic circuits
- PMID: 20829787
- PMCID: PMC4100692
- DOI: 10.1038/nature09326
Functional roles for noise in genetic circuits
Abstract
The genetic circuits that regulate cellular functions are subject to stochastic fluctuations, or 'noise', in the levels of their components. Noise, far from just a nuisance, has begun to be appreciated for its essential role in key cellular activities. Noise functions in both microbial and eukaryotic cells, in multicellular development, and in evolution. It enables coordination of gene expression across large regulons, as well as probabilistic differentiation strategies that function across cell populations. At the longest timescales, noise may facilitate evolutionary transitions. Here we review examples and emerging principles that connect noise, the architecture of the gene circuits in which it is present, and the biological functions it enables. We further indicate some of the important challenges and opportunities going forward.
Figures
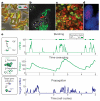
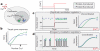
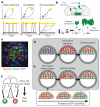
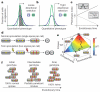
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

