MEC-17 is an alpha-tubulin acetyltransferase
- PMID: 20829795
- PMCID: PMC2938957
- DOI: 10.1038/nature09324
MEC-17 is an alpha-tubulin acetyltransferase
Abstract
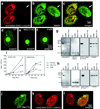
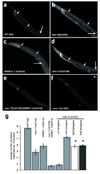
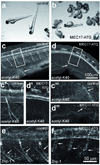
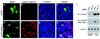
In most eukaryotic cells, subsets of microtubules are adapted for specific functions by post-translational modifications (PTMs) of tubulin subunits. Acetylation of the epsilon-amino group of K40 on alpha-tubulin is a conserved PTM on the luminal side of microtubules that was discovered in the flagella of Chlamydomonas reinhardtii. Studies on the significance of microtubule acetylation have been limited by the undefined status of the alpha-tubulin acetyltransferase. Here we show that MEC-17, a protein related to the Gcn5 histone acetyltransferases and required for the function of touch receptor neurons in Caenorhabditis elegans, acts as a K40-specific acetyltransferase for alpha-tubulin. In vitro, MEC-17 exclusively acetylates K40 of alpha-tubulin. Disruption of the Tetrahymena MEC-17 gene phenocopies the K40R alpha-tubulin mutation and makes microtubules more labile. Depletion of MEC-17 in zebrafish produces phenotypes consistent with neuromuscular defects. In C. elegans, MEC-17 and its paralogue W06B11.1 are redundantly required for acetylation of MEC-12 alpha-tubulin, and contribute to the function of touch receptor neurons partly via MEC-12 acetylation and partly via another function, possibly by acetylating another protein. In summary, we identify MEC-17 as an enzyme that acetylates the K40 residue of alpha-tubulin, the only PTM known to occur on the luminal surface of microtubules.
Figures
References
-
- Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. - PubMed
-
- Steczkiewicz K, Kinch L, Grishin NV, Rychlewski L, Ginalski K. Eukaryotic domain of unknown function DUF738 belongs to Gcn5-related N-acetyltransferase superfamily. Cell Cycle. 2006;5:2927–2930. - PubMed
-
- Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science. 1989;243:1027–1033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

