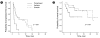Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer
- PMID: 20830227
- PMCID: PMC2932943
- DOI: 10.3904/kjim.2010.25.3.294
Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer
Abstract
Background/aims: The efficacy and safety of pemetrexed, gefitinib, and erlotinib administration in previously treated patients with non-small cell lung cancer (NSCLC) were compared.
Methods: THE STUDY PATIENTS MET THE FOLLOWING CRITERIA: histologically confirmed, previously treated advanced (stage IIIB or IV) or recurrent NSCLC; a measurable lesion; ≥ 18 years of age; Eastern Cooperative Oncology Group Performance status 0 to 2; and no prior exposure to the three study drugs. Patients received 500 mg/m(2) of pemetrexed intravenously every 3 weeks with vitamin supplementation, gefitinib (250 mg/day per os), or erlotinib (150 mg/day per os).
Results: Of 57 patients (pemetrexed, 20; gefitinib, 20; and erlotinib, 17), 55 were evaluated for a response. The numbers of males, smokers, and squamous histology were increased in the pemetrexed group compared to the other groups. The objective response rates were 5.3%, 25.0%, and 12.5% (p = 0.22), and the disease control rates (DCR) were 5.3%, 40.0%, and 50.0%, respectively (p < 0.01). The median progression-free survival (PFS) was 1.7, 3.5, and 4.4 months (p < 0.01) and the median overall survival (OS) was 5.6, 21.8, and 21.5 months (p = 0.04), respectively. In subgroup analyses, patients with non-squamous histology, males, and a smoking history had a higher DCR and longer PFS with gefitinib and erlotinib than with pemetrexed. All three chemotherapeutic agents had manageable toxicities.
Conclusions: Both oral epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) had comparable efficacy and safety. The superior PFS and OS of EGFR TKIs with more favorable baseline clinical characteristics than those of pemetrexed suggest the impact of baseline clinicopathological factors.
Keywords: Erlotinib; Lung neoplasms; Pemetrexed; efitinib.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol. 2008;38:327–333. - PubMed
-
- Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Stinchcombe TE, Socinski MA. Considerations for second-line therapy of non-small cell lung cancer. Oncologist. 2008;13(Suppl 1):28–36. - PubMed
-
- Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–130. - PubMed
-
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous