Potential role for MATER in cytoplasmic lattice formation in murine oocytes
- PMID: 20830304
- PMCID: PMC2935378
- DOI: 10.1371/journal.pone.0012587
Potential role for MATER in cytoplasmic lattice formation in murine oocytes
Abstract
Background: Mater and Padi6 are maternal effect genes that are first expressed during oocyte growth and are required for embryonic development beyond the two-cell stage in the mouse. We have recently found that PADI6 localizes to, and is required for the formation of, abundant fibrillar Triton X-100 (Triton) insoluble structures termed the oocyte cytoplasmic lattices (CPLs). Given their similar expression profiles and mutant mouse phenotypes, we have been testing the hypothesis that MATER also plays a role in CPL formation and/or function.
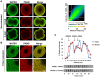
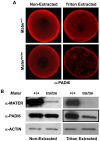
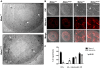
Methodology/findings: Herein, we show that PADI6 and MATER co-localize throughout the oocyte cytoplasm following Triton extraction, suggesting that MATER co-localizes with PADI6 at the CPLs. Additionally, the solubility of PADI6 was dramatically increased in Mater(tm/tm) oocytes following Triton extraction, suggesting that MATER is involved in CPL nucleation. This prediction is supported by transmission electron microscopic analysis of Mater(+/+) and Mater(tm/tm) germinal vesicle stage oocytes which illustrated that volume fraction of CPLs was reduced by 90% in Mater(tm/tm) oocytes compared to Mater(+/+) oocytes.
Conclusions: Taken together, these results suggest that, similar to PADI6, MATER is also required for CPL formation. Given that PADI6 and MATER are essential for female fertility, these results not only strengthen the hypothesis that the lattices play a critical role in mediating events during the oocyte-to-embryo transition but also increase our understanding of the molecular nature of the CPLs.
Conflict of interest statement
Figures

Similar articles
-
Role for PADI6 in securing the mRNA-MSY2 complex to the oocyte cytoplasmic lattices.Cell Cycle. 2017 Feb 16;16(4):360-366. doi: 10.1080/15384101.2016.1261225. Epub 2016 Dec 8. Cell Cycle. 2017. PMID: 27929740 Free PMC article.
-
Subcellular localization of cytoplasmic lattice-associated proteins is dependent upon fixation and processing procedures.PLoS One. 2011 Feb 16;6(2):e17226. doi: 10.1371/journal.pone.0017226. PLoS One. 2011. PMID: 21359190 Free PMC article.
-
Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo.Development. 2008 Aug;135(15):2627-36. doi: 10.1242/dev.016329. Epub 2008 Jul 3. Development. 2008. PMID: 18599511 Free PMC article.
-
PADI6: What we know about the elusive fifth member of the peptidyl arginine deiminase family.Philos Trans R Soc Lond B Biol Sci. 2023 Nov 20;378(1890):20220242. doi: 10.1098/rstb.2022.0242. Epub 2023 Oct 2. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37778376 Free PMC article. Review.
-
Use of proteomics to identify highly abundant maternal factors that drive the egg-to-embryo transition.Reproduction. 2010 May;139(5):809-23. doi: 10.1530/REP-09-0538. Epub 2010 Jan 27. Reproduction. 2010. PMID: 20106898 Review.
Cited by
-
Cytoplasmic lattices are not linked to mouse 2-cell embryos developmental arrest.Eur J Histochem. 2018 Sep 24;62(4):2972. doi: 10.4081/ejh.2018.2972. Eur J Histochem. 2018. PMID: 30362672 Free PMC article.
-
The role of MATER in endoplasmic reticulum distribution and calcium homeostasis in mouse oocytes.Dev Biol. 2014 Feb 15;386(2):331-9. doi: 10.1016/j.ydbio.2013.12.025. Epub 2013 Dec 25. Dev Biol. 2014. PMID: 24374158 Free PMC article.
-
ECAT1 is essential for human oocyte maturation and pre-implantation development of the resulting embryos.Sci Rep. 2016 Dec 5;6:38192. doi: 10.1038/srep38192. Sci Rep. 2016. PMID: 27917907 Free PMC article.
-
Dynamic changes in leptin distribution in the progression from ovum to blastocyst of the pre-implantation mouse embryo.Reproduction. 2011 Jun;141(6):767-77. doi: 10.1530/REP-10-0532. Epub 2011 Mar 28. Reproduction. 2011. PMID: 21444625 Free PMC article.
-
Post-transcriptional control of gene expression in mouse early embryo development: a view from the tip of the iceberg.Genes (Basel). 2011 Apr 6;2(2):345-59. doi: 10.3390/genes2020345. Genes (Basel). 2011. PMID: 24710195 Free PMC article.
References
-
- Bachvarova R. Gene expression during oogenesis and oocyte development in mammals. Dev Biol (N Y 1985) 1985;1:453–524. - PubMed
-
- Schultz RM. Regulation of zygotic gene activation in the mouse. Bioessays. 1993;15(8):531–538. - PubMed
-
- Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6(1):117–131. - PubMed
-
- Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6(1):133–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

