Use of recombinant cell-permeable small peptides to modulate glucocorticoid sensitivity of acute lymphoblastic leukemia cells
- PMID: 20831260
- PMCID: PMC2953583
- DOI: 10.1021/bi1007723
Use of recombinant cell-permeable small peptides to modulate glucocorticoid sensitivity of acute lymphoblastic leukemia cells
Abstract
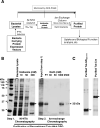
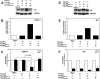
Glucocorticoid (GC) hormones induce apoptosis in T-cell and pre-B-cell acute lymphoblastic leukemia (ALL) cells. Steroid-mediated apoptosis requires a threshold level of the glucocorticoid receptor (GR) protein, and increasing the intracellular GR levels in ALL cells would augment their hormone sensitivity. A protein transduction domain (PTD) approach was used to accomplish this. We produced an HIV Tat PTD domain fusion protein (Tat-GR(554-777)) that potentially competes for the degradation of GR protein by the ubiquitin-proteasome system and should thus increase its intracellular levels by "stabilizing" the GR. We also designed a fusion peptide for the c-Myb DNA binding domain, Tat-c-Myb DBD, since the biological function of this peptide as a dominant negative inhibitor of the c-Myb protein was already known. Purified, bacterially expressed Tat-c-Myb DBD and Tat-GR(554-777) exhibited highly efficient transduction into cultured ALL cell lines including 697 (pre-B-ALL) and CEM-C7 (T-ALL) cells. As expected, the transduced Tat-c-Myb DBD peptide inhibited steroid-mediated stimulation of a GR promoter-luciferase reporter gene. Significantly, transduced Tat-GR(554-777) effectively increased intracellular GR levels in the GC-resistant T-ALL cell line, CEM-C1, and in the pre-B-ALL 697 cell line. Furthermore, transduction of Tat-GR(554-777) rendered GC-resistant CEM-C1 cells sensitive to steroid killing and further sensitized 697 cells to steroid. The use of Tat-fusion peptide transduction may eventually lead to innovative therapeutic modalities to improve the clinical response of patients suffering from T-cell and pre-B-cell acute lymphoblastic leukemia by increasing steroid responsiveness and perhaps converting steroid-resistant leukemia to a hormone-responsive phenotype.
Figures



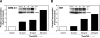

Similar articles
-
Resistance of human leukemic CEM-C1 cells is overcome by synergism between glucocorticoid and protein kinase A pathways: correlation with c-Myc suppression.Cancer Res. 1998 Aug 15;58(16):3684-93. Cancer Res. 1998. PMID: 9721879
-
c-Myb interacts with the glucocorticoid receptor and regulates its level in pre-B-acute lymphoblastic leukemia cells.Mol Cell Endocrinol. 2012 Sep 25;361(1-2):124-32. doi: 10.1016/j.mce.2012.03.024. Epub 2012 Apr 10. Mol Cell Endocrinol. 2012. PMID: 22516378 Free PMC article.
-
A new, lineage specific, autoup-regulation mechanism for human glucocorticoid receptor gene expression in 697 pre-B-acute lymphoblastic leukemia cells.Mol Endocrinol. 2011 Jan;25(1):44-57. doi: 10.1210/me.2010-0249. Epub 2010 Nov 17. Mol Endocrinol. 2011. PMID: 21084380 Free PMC article.
-
Regulation of a distinctive set of genes in glucocorticoid-evoked apoptosis in CEM human lymphoid cells.Recent Prog Horm Res. 2003;58:175-97. doi: 10.1210/rp.58.1.175. Recent Prog Horm Res. 2003. PMID: 12795419 Review.
-
Resistance to glucocorticoid-induced apoptosis in lymphoblastic leukemia.J Endocrinol. 2003 Jul;178(1):19-27. doi: 10.1677/joe.0.1780019. J Endocrinol. 2003. PMID: 12844332 Review.
Cited by
-
Emerging role of MYB transcription factors in cancer drug resistance.Cancer Drug Resist. 2024 Apr 30;7:15. doi: 10.20517/cdr.2023.158. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38835346 Free PMC article. Review.
References
-
- Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J. Natl. Cancer Inst. 2008;100:1301–1309. - PubMed
-
- DeAngelo DJ. The treatment of adolescents and young adults with acute lymphoblastic leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2005;2005:123–130. - PubMed
-
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006;354:166–178. - PubMed
-
- Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N. Engl. J. Med. 2004;350:1535–1548. - PubMed
-
- Rivera GK, Pinkel D, Simone JV, Hancock ML, Crist WM. Treatment of acute lymphoblastic leukemia -- 30 years’ experience at St. Jude Children's Research Hospital. N. Engl. J. Med. 1993;329:1289–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

