Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice
- PMID: 20831751
- PMCID: PMC3225007
- DOI: 10.1111/j.1528-1167.2010.02592.x
Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice
Abstract
Purpose: KCNJ10 encodes subunits of inward rectifying potassium (Kir) channel Kir4.1 found predominantly in glial cells within the brain. Genetic inactivation of these channels in glia impairs extracellular K(+) and glutamate clearance and produces a seizure phenotype. In both mice and humans, polymorphisms and mutations in the KCNJ10 gene have been associated with seizure susceptibility. The purpose of the present study was to determine whether there are differences in Kir channel activity and potassium- and glutamate-buffering capabilities between astrocytes from seizure resistant C57BL/6 (B6) and seizure susceptible DBA/2 (D2) mice that are consistent with an altered K(+) channel activity as a result of genetic polymorphism of KCNJ10.
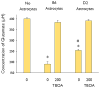
Methods: Using cultured astrocytes and hippocampal brain slices together with whole-cell patch-clamp, we determined the electrophysiologic properties, particularly K(+) conductances, of B6 and D2 mouse astrocytes. Using a colorimetric assay, we determined glutamate clearance capacity by B6 and D2 astrocytes.
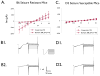
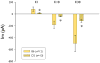
Results: Barium-sensitive Kir currents elicited from B6 astrocytes are substantially larger than those elicited from D2 astrocytes. In addition, potassium and glutamate buffering by D2 cortical astrocytes is impaired, relative to buffering by B6 astrocytes.
Discussion: In summary, the activity of Kir4.1 channels differs between seizure-susceptible D2 and seizure-resistant B6 mice. Reduced activity of Kir4.1 channels in astrocytes of D2 mice is associated with deficits in potassium and glutamate buffering. These deficits may, in part, explain the relatively low seizure threshold of D2 mice.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures

References
-
- Abe K, Abe Y, Saito H. Evaluation of L-glutamate clearance capacity of cultured rat cortical astrocytes. Biol Pharm Bull. 2000;23:204–207. - PubMed
-
- Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, Ataxia, Sensorineural Deafness, Tubulopathy, and KCNJ10 Mutations. New Eng J Med. 2009;360:1960–1970. - PMC - PubMed
-
- Broberg M, Pope KJ, Lewis T, Olsson T, Nilsson M, Willoughhby JO. Cell swelling precedes seizures induced by inhibition of astrocytic metabolism. Epilepsy Res. 2008;80:132–141. - PubMed
-
- Buono RJ, Lohoff FW, Sander T, Sperling MR, O’Connor MJ, Dlugos DJ, Ryan SG, Golden GT, Zhao H, Scattergood TM, Berrettini WH, Ferraro TN. Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res. 2004;58:175–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

