The Belgian MIRA (MabThera In Rheumatoid Arthritis) registry: clues for the optimization of rituximab treatment strategies
- PMID: 20831776
- PMCID: PMC2990996
- DOI: 10.1186/ar3129
The Belgian MIRA (MabThera In Rheumatoid Arthritis) registry: clues for the optimization of rituximab treatment strategies
Abstract
Introduction: This study describes the results of the Belgian 'MabThera In Rheumatoid Arthritis (MIRA)' registry: effectiveness, safety and evaluation of the current retreatment practice on the background of the Belgian reimbursement criteria for rituximab.
Methods: All Belgian rheumatologists had the possibility to participate in the study. Patients entered the registry in November 2006 and the entry is still open.
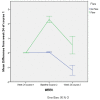
Results: By mid-September 2009, 401 patients had entered the registry with a mean follow-up time of 70 weeks. Overall, DAS28-ESR decreased from 6.0 at baseline to 4.2 at week 16. Further decrease of disease activity was observed at the end of year 1 and year 2 with mean DAS28-ESR of 4.0 and 3.7 at these respective time points. More than 80% of patients showed a EULAR response at week 16. Patients could be retreated if they had DAS scores of > 3.2 at least 6 months after the previous course. Second and third courses were given in 224 and 104 patients, respectively. At month 6 after the second course, significantly lower DAS28-ESR values were observed compared to the first course. This was especially the case for patients who were retreated before they showed an obvious flare (DAS increase > 1.2).
Conclusions: This study describes the follow-up of a daily clinical practice cohort of 401 RA patients with long-standing refractory disease treated with rituximab. Relatively high DAS28 values at the start of each retreatment, compared to values 6 months after each treatment course, were noted. Moreover, further decrease of DAS28 scores after the second course was significantly more pronounced in those patients who didn't show an obvious flare. These two elements suggest that treatment of RA patients with rituximab could be optimized by earlier retreatment.
Figures

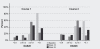
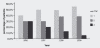
References
-
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. - DOI - PubMed
-
- Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, Burge DJ. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:353–363. doi: 10.1002/art.20019. - DOI - PubMed
-
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. - DOI - PubMed
-
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

