Leucine-rich alpha-2-glycoprotein-1 is upregulated in sera and tumors of ovarian cancer patients
- PMID: 20831812
- PMCID: PMC2949730
- DOI: 10.1186/1757-2215-3-21
Leucine-rich alpha-2-glycoprotein-1 is upregulated in sera and tumors of ovarian cancer patients
Abstract
Background: New biomarkers that replace or are used in conjunction with the current ovarian cancer diagnostic antigen, CA125, are needed for detection of ovarian cancer in the presurgical setting, as well as for detection of disease recurrence. We previously demonstrated the upregulation of leucine-rich alpha-2-glycoprotein-1 (LRG1) in the sera of ovarian cancer patients compared to healthy women using quantitative mass spectrometry.
Methods: LRG1 was quantified by ELISA in serum from two relatively large cohorts of women with ovarian cancer and benign gynecological disease. The expression of LRG1 in ovarian cancer tissues and cell lines was examined by gene microarray, reverse-transcriptase polymerase chain reaction (RT-PCR), Western blot, immunocytochemistry and mass spectrometry.
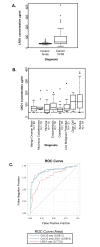
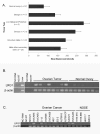
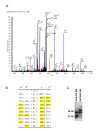
Results: Mean serum LRG1 was higher in 58 ovarian cancer patients than in 56 healthy women (89.33 ± 77.90 vs. 42.99 ± 9.88 ug/ml; p = 0.0008) and was highest among stage III/IV patients. In a separate set of 193 pre-surgical samples, LRG1 was higher in patients with serous or clear cell ovarian cancer (145.82 ± 65.99 ug/ml) compared to patients with benign gynecological diseases (82.53 ± 76.67 ug/ml, p < 0.0001). CA125 and LRG1 levels were moderately correlated (r = 0.47, p < 0.0001). LRG1 mRNA levels were higher in ovarian cancer tissues and cell lines compared to their normal counterparts when analyzed by gene microarray and RT-PCR. LRG1 protein was detected in ovarian cancer tissue samples and cell lines by immunocytochemistry and Western blotting. Multiple iosforms of LRG1 were observed by Western blot and were shown to represent different glycosylation states by digestion with glycosidase. LRG1 protein was also detected in the conditioned media of ovarian cancer cell culture by ELISA, Western blotting, and mass spectrometry.
Conclusions: Serum LRG1 was significantly elevated in women with ovarian cancer compared to healthy women and women with benign gynecological disease, and was only moderately correlated with CA125. Ovarian cancer cells secrete LRG1 and may contribute directly to the elevated levels of LRG1 observed in the serum of ovarian cancer patients. Future studies will determine whether LRG1 may serve as a biomarker for presurgical diagnosis, disease recurrence, and/or as a target for therapy.
Figures

References
-
- Ries L, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Eisner MP, Horner MJ, Howlader N, Hayat M, Hankey BF, Edwards BK. SEER Cancer Statistics Review, 1975-2003. Book SEER Cancer Statistics Review, 1975-2003 (Editor ed.^eds.). City. 2005.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

