Evaluation of multicomponent recombinant vaccines against Actinobacillus pleuropneumoniae in mice
- PMID: 20831818
- PMCID: PMC2944310
- DOI: 10.1186/1751-0147-52-52
Evaluation of multicomponent recombinant vaccines against Actinobacillus pleuropneumoniae in mice
Abstract
Background: Porcine contagious pleuropneumonia (PCP) is a highly contagious disease that is caused by Actinobacillus pleuropneumoniae (APP) and characterized by severe fibrinous necrotizing hemorrhagic pleuropneumonia, which is a severe threat to the swine industry. In addition to APP RTX-toxins I (ApxI), APP RTX-toxin II (ApxII), APP RTX-toxin III (ApxIII) and Outer membrane protein (OMP), there may be other useful antigens that can contribute to protection. In the development of an efficacious vaccine against APP, the immunogenicities of multicomponent recombinant subunit vaccines were evaluated.
Methods: Six major virulent factor genes of APP, i.e., apxI, apxII, apxIII, APP RTX-toxins IV (apxIV), omp and type 4 fimbrial structural (apfa) were expressed. BALB/c mice were immunized with recombinant ApxI ( rApxI), recombinant ApxII (rApxII), recombinant ApxIII (rApxIII) and recombinant OMP (rOMP) (Group I); rApxI, rApxII, rApxIII, recombinant ApxIV (rApxIV), recombinant Apfa (rApfa) and rOMP (Group II); APP serotype 1 (APP1) inactivated vaccine (Group III); or phosphate-buffered saline (PBS) (Control group), respectively. After the first immunization, mice were subjected to two booster immunizations at 2-week intervals, followed by challenge with APP1 Shope 4074 and APP2 S1536.
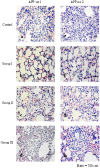
Results: The efficacy of the multicomponent recombinant subunit vaccines was evaluated on the basis of antibody titers, survival rates, lung lesions and indirect immunofluorescence (IIF) detection of APP. The antibody level of Group I was significantly higher than those of the other three groups (P < 0.05). The survival rate of Group I was higher than that of Groups II and III (P < 0.05) and the control (P < 0.01). Compared with the other three groups, the lungs of Group I did not exhibit obvious hemorrhage or necrosis, and only showed weak and scattered fluorescent dots by IIF detection.
Conclusion: The result indicates that the multicomponent recombinant subunit vaccine composed of rApxI, rApxII, rApxIII and rOMP can provide effective cross-protection against homologous and heterologous APP challenge.
Figures

References
-
- Stine DL, Fedorka-Cray PJ, Huether MJ, Gentry MJ, Anderson GA. Comparison of serum responses in swine after vaccination and challenge exposure with Actinobacillus pleuropneumoniae serotype 1. Am J Vet Res. 1994;55:1238–1243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

