Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors
- PMID: 20832340
- PMCID: PMC3063338
- DOI: 10.1016/j.immuni.2010.08.012
Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors
Abstract
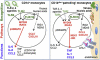
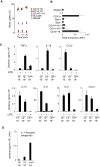
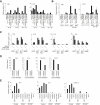
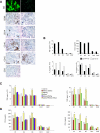
Monocytes are effectors of the inflammatory response to microbes. Human CD14(+) monocytes specialize in phagocytosis and production of reactive oxygen species and secrete inflammatory cytokines in response to a broad range of microbial cues. Here, we have characterized the functions of human monocytes that lack CD14 (CD14(dim)) and express CD16. CD14(dim) monocytes were genetically distinct from natural killer cells. Gene expression analyses indicated similarities with murine patrolling Gr1(dim) monocytes, and they patrolled the endothelium of blood vessels after adoptive transfer, in a lymphocyte function-associated antigen-1-dependent manner. CD14(dim) monocytes were weak phagocytes and did not produce ROS or cytokines in response to cell-surface Toll-like receptors. Instead, they selectively produced TNF-α, IL-1β, and CCL3 in response to viruses and immune complexes containing nucleic acids, via a proinflammatory TLR7-TLR 8-MyD88-MEK pathway. Thus, CD14(dim) cells are bona fide monocytes involved in the innate local surveillance of tissues and the pathogenesis of autoimmune diseases.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Diversity: a hallmark of monocyte society.Immunity. 2010 Sep 24;33(3):289-91. doi: 10.1016/j.immuni.2010.09.007. Immunity. 2010. PMID: 20870168
References
-
- Agrawal A., Dillon S., Denning T.L., Pulendran B. ERK1-/- mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:5788–5796. - PubMed
-
- Ancuta P., Kunstman K.J., Autissier P., Zaman T., Stone D., Wolinsky S.M., Gabuzda D. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–276. - PubMed
-
- Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

