Brk/PTK6 signaling in normal and cancer cell models
- PMID: 20832360
- PMCID: PMC2981671
- DOI: 10.1016/j.coph.2010.08.007
Brk/PTK6 signaling in normal and cancer cell models
Abstract
Breast tumor kinase (Brk), also termed PTK6, is known to function in cell-type and context-dependent processes governing normal differentiation. However, in tumors in which Brk is overexpressed, this unusual soluble tyrosine kinase is emerging as a mediator of cancer cell phenotypes, including increased proliferation, survival, and migration. Nuclear and cytoplasmic substrates phosphorylated by Brk include a collection of regulatory RNA-binding proteins, adaptor molecules that link Brk to signaling pathways generally associated with the activation of growth factor receptors, and Signal Transducers and Activators of Transcription (STAT) molecules that are direct regulators of gene expression. Understanding Brk-dependent regulation of these key signaling pathways and how they influence cancer cell behavior is predicted to inform the development of improved 'targeted' cancer therapies and may provide insight into ways to avoid chemo-resistance to established treatments.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

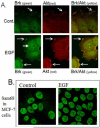
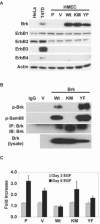
References
Key References
-
- Kamalati T, Jolin HE, Mitchell PJ, Barker KT, Jackson LE, Dean CJ, Page MJ, Gusterson BA, Crompton MR. Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J Biol Chem. 1996;271(48):30956–30963. - PubMed
-
This was the first report to show that Brk interacted with ErbB family members, and that Brk expression could enhance proliferation of mammary epithelial cells.
-
- Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004;24(24):10558–10572. - PMC - PubMed
-
Chen et al. were the first to show that Brk expression can enhance cellular migration and invasion. This group was also the first to demonstrate that paxillin is a Brk substrate and Brk-induced phosphorylation of paxillin promotes Rac activation.
-
- Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67(9):4199–4209. - PubMed
-
Ostrander et al, were the first to show that Brk kinase activity is actived by HRG, and Brk expression is required for HRG-induced activation of p38 MAPK and ERK5. It was found that the Brk/Rac1/p38 MAPK pathway is required for EGF and HRG-induced breast cancer cell migration.
-
-
Haegebarth A, Bie W, Yang R, Crawford SE, Vasioukhin V, Fuchs E, Tyner AL. Protein tyrosine kinase 6 negatively regulates growth and promotes enterocyte differentiation in the small intestine. Mol Cell Biol. 2006;26(13):4949–4957. AND Haegebarth A, Perekatt AO, Bie W, Gierut JJ, Tyner AL. Induction of protein tyrosine kinase 6 in mouse intestinal crypt epithelial cells promotes DNA damage-induced apoptosis. Gastroenterology. 2009;137(3):945–954. These two papers clearly define a role for Brk in normal epithelial cell biology that is clearly different to what is observed in cancer models. The PTK6 knockout mice display increased proliferation and enhanced Akt activation in the small intestine. Loss of PTK6 also leads to a decrease in expression of specific markers of differentiation. Furthermore, when the PTK6 knockout mice are challenged with irradiation, it was found the PTK6 expression enhances DNA damage-induced apoptosis.
-
References
-
- Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, Gusterson BA, Crompton MR. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9(8):2383–2390. - PubMed
-
- Lee ST, Strunk KM, Spritz RA. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene. 1993;8(12):3403–3410. - PubMed
-
- Siyanova EY, Serfas MS, Mazo IA, Tyner AL. Tyrosine kinase gene expression in the mouse small intestine. Oncogene. 1994;9(7):2053–2057. - PubMed
-
- Vasioukhin V, Serfas MS, Siyanova EY, Polonskaia M, Costigan VJ, Liu B, Thomason A, Tyner AL. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene. 1995;10(2):349–357. - PubMed
-
- Lee H, Kim M, Lee KH, Kang KN, Lee ST. Exon-intron structure of the human PTK6 gene demonstrates that PTK6 constitutes a distinct family of non-receptor tyrosine kinase. Mol Cells. 1998;8(4):401–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

