Basic and translational applications of engineered MHC class I proteins
- PMID: 20832361
- PMCID: PMC2949479
- DOI: 10.1016/j.it.2010.07.003
Basic and translational applications of engineered MHC class I proteins
Abstract
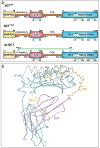
Major histocompatibility complex (MHC) class I molecules can be engineered as single chain trimers (SCTs) that sequentially incorporate all three subunits of the fully assembled proteins, namely peptide, β2 microglobulin, and heavy chain. SCTs have been made with many different MHC-peptide complexes and are used as novel diagnostic and therapeutic reagents, as well as probes for diverse biological questions. Here, we review the recent and diverse applications of SCTs. These applications include new approaches to enumerate disease-related T cells, DNA vaccines, eliciting responses to pre-assembled MHC-peptide complexes, and unique probes of lymphocyte development and activation. Future applications of SCTs will be driven by their further engineering and the ever-expanding identification of disease-related peptides using chemical, genetic and computational approaches.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Applications of major histocompatibility complex class I molecules expressed as single chains.Immunol Res. 2005;32(1-3):109-21. doi: 10.1385/ir:32:1-3:109. Immunol Res. 2005. PMID: 16106063 Review.
-
Preparation of stable single-chain trimers engineered with peptide, beta2 microglobulin, and MHC heavy chain.Curr Protoc Immunol. 2009 Nov;Chapter 17:17.5.1-17.5.17. doi: 10.1002/0471142735.im1705s87. Curr Protoc Immunol. 2009. PMID: 19918946
-
Single chain MHC I trimer-based DNA vaccines for protection against Listeria monocytogenes infection.Vaccine. 2012 Mar 9;30(12):2178-86. doi: 10.1016/j.vaccine.2012.01.012. Epub 2012 Jan 26. Vaccine. 2012. PMID: 22285270 Free PMC article.
-
A mutant human beta2-microglobulin can be used to generate diverse multimeric class I peptide complexes as specific probes for T cell receptors.J Immunol Methods. 1998 May 1;214(1-2):41-50. doi: 10.1016/s0022-1759(98)00035-0. J Immunol Methods. 1998. PMID: 9692857
-
Properties and applications of single-chain major histocompatibility complex class I molecules.Antioxid Redox Signal. 2011 Aug 1;15(3):645-55. doi: 10.1089/ars.2010.3694. Epub 2011 Mar 31. Antioxid Redox Signal. 2011. PMID: 21126187 Free PMC article. Review.
Cited by
-
Donor-unrestricted T cells in the human CD1 system.Immunogenetics. 2016 Aug;68(8):577-96. doi: 10.1007/s00251-016-0942-x. Epub 2016 Aug 9. Immunogenetics. 2016. PMID: 27502318 Free PMC article. Review.
-
β2-Microglobulin-mediated signaling as a target for cancer therapy.Anticancer Agents Med Chem. 2014 Mar;14(3):343-52. doi: 10.2174/18715206113139990092. Anticancer Agents Med Chem. 2014. PMID: 23848204 Free PMC article. Review.
-
The Role of MHC-E in T Cell Immunity Is Conserved among Humans, Rhesus Macaques, and Cynomolgus Macaques.J Immunol. 2018 Jan 1;200(1):49-60. doi: 10.4049/jimmunol.1700841. Epub 2017 Nov 17. J Immunol. 2018. PMID: 29150562 Free PMC article.
-
HLA-E-restricted, Gag-specific CD8+ T cells can suppress HIV-1 infection, offering vaccine opportunities.Sci Immunol. 2021 Mar 25;6(57):eabg1703. doi: 10.1126/sciimmunol.abg1703. Sci Immunol. 2021. PMID: 33766848 Free PMC article.
-
Structural and functional characterization of a single-chain peptide-MHC molecule that modulates both naive and activated CD8+ T cells.Proc Natl Acad Sci U S A. 2011 Aug 16;108(33):13682-7. doi: 10.1073/pnas.1110971108. Epub 2011 Aug 8. Proc Natl Acad Sci U S A. 2011. PMID: 21825122 Free PMC article.
References
-
- Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. - PubMed
-
- Chapatte L, et al. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 2006;66:5461–5468. - PubMed
-
- Greten TF, et al. Peptide-beta2-microglobulin-MHC fusion molecules bind antigen-specific T cells and can be used for multivalent MHC-Ig complexes. J Immunol Methods. 2002;271:125–135. - PubMed
-
- Yu YY, et al. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168:3145–3149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

