Rictor forms a complex with Cullin-1 to promote SGK1 ubiquitination and destruction
- PMID: 20832730
- PMCID: PMC2939073
- DOI: 10.1016/j.molcel.2010.08.016
Rictor forms a complex with Cullin-1 to promote SGK1 ubiquitination and destruction
Abstract
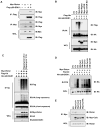
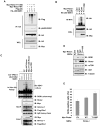
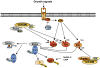
The Rictor/mTOR complex (also known as mTORC2) plays a critical role in cellular homeostasis by phosphorylating AGC kinases such as Akt and SGK at their hydrophobic motifs to activate downstream signaling. However, the regulation of mTORC2 and whether it has additional function(s) remain largely unknown. Here, we report that Rictor associates with Cullin-1 to form a functional E3 ubiquitin ligase. Rictor, but not Raptor or mTOR alone, promotes SGK1 ubiquitination. Loss of Rictor/Cullin-1-mediated ubiquitination leads to increased SGK1 protein levels as detected in Rictor null cells. Moreover, as part of a feedback mechanism, phosphorylation of Rictor at T1135 by multiple AGC kinases disrupts the interaction between Rictor and Cullin-1 to impair SGK1 ubiquitination. These findings indicate that the Rictor/Cullin-1 E3 ligase activity is regulated by a specific signal relay cascade and that misregulation of this mechanism may contribute to the frequent overexpression of SGK1 in various human cancers.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. - PubMed
-
- Bogusz AM, Brickley DR, Pew T, Conzen SD. A novel N-terminal hydrophobic motif mediates constitutive degradation of serum- and glucocorticoid-induced kinase-1 by the ubiquitin-proteasome pathway. Febs J. 2006;273:2913–2928. - PubMed
-
- Brickley DR, Mikosz CA, Hagan CR, Conzen SD. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1) J Biol Chem. 2002;277:43064–43070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

