Solution NMR structure of the V27A drug resistant mutant of influenza A M2 channel
- PMID: 20833142
- PMCID: PMC3215091
- DOI: 10.1016/j.bbrc.2010.09.008
Solution NMR structure of the V27A drug resistant mutant of influenza A M2 channel
Abstract
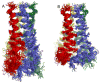
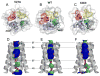
The M2 protein of influenza A virus forms a proton-selective channel that is required for viral replication. It is the target of the anti-influenza drugs, amantadine and rimantadine. Widespread drug resistant mutants, however, has greatly compromised the effectiveness of these drugs. Here, we report the solution NMR structure of the highly pathogenic, drug resistant mutant V27A. The structure reveals subtle structural differences from wildtype that maybe linked to drug resistance. The V27A mutation significantly decreases hydrophobic packing between the N-terminal ends of the transmembrane helices, which explains the looser, more dynamic tetrameric assembly. The weakened channel assembly can resist drug binding either by destabilizing the rimantadine-binding pocket at Asp44, in the case of the allosteric inhibition model, or by reducing hydrophobic contacts with amantadine in the pore, in the case of the pore-blocking model. Moreover, the V27A structure shows a substantially increased channel opening at the N-terminal end, which may explain the faster proton conduction observed for this mutant. Furthermore, due to the high quality NMR data recorded for the V27A mutant, we were able to determine the structured region connecting the channel domain to the C-terminal amphipathic helices that was not determined in the wildtype structure. The new structural data show that the amphipathic helices are packed much more closely to the channel domain and provide new insights into the proton transfer pathway.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


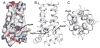
References
-
- Helenius A. Unpacking the incoming influenza virus. Cell. 1992;69:577–578. - PubMed
-
- Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–28. - PubMed
-
- Davies WL, Grunert RR, Haff RF, McGahen JW, Neumayer EM, Paulshock M, Watts JC, Wood TR, Hermann EC, Hoffmann CE. Antiviral Activity of 1-Adamantanamine (Amantadine) Science. 1964;144:862–3. - PubMed
-
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. Jama. 2006;295:891–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

