Biochemical characterization of Plasmodium falciparum dipeptidyl aminopeptidase 1
- PMID: 20833209
- PMCID: PMC3514014
- DOI: 10.1016/j.molbiopara.2010.08.004
Biochemical characterization of Plasmodium falciparum dipeptidyl aminopeptidase 1
Abstract
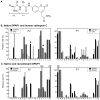
Dipeptidyl aminopeptidase 1 (DPAP1) is an essential food vacuole enzyme with a putative role in hemoglobin catabolism by the erythrocytic malaria parasite. Here, the biochemical properties of DPAP1 have been investigated and compared to those of the human ortholog cathepsin C. To facilitate the characterization of DPAP1, we have developed a method for the production of purified recombinant DPAP1 with properties closely resembling those of the native enzyme. Like cathepsin C, DPAP1 is a chloride-activated enzyme that is most efficient in catalyzing amide bond hydrolysis at acidic pH values. The monomeric quaternary structure of DPAP1 differs from the homotetrameric structure of cathepsin C, which suggests that tetramerization is required for a cathepsin C-specific function. The S1 and S2 subsite preferences of DPAP1 and cathepsin C were profiled with a positional scanning synthetic combinatorial library. The S1 preferences bore close similarity to those of other C1-family cysteine peptidases. The S2 subsites of both DPAP1 and cathepsin C accepted aliphatic hydrophobic residues, proline, and some polar residues, yielding a distinct specificity profile. DPAP1 efficiently catalyzed the hydrolysis of several fluorogenic dipeptide substrates; surprisingly, however, a potential substrate with a P2-phenylalanine residue was instead a competitive inhibitor. Together, our biochemical data suggest that DPAP1 accelerates the production of amino acids from hemoglobin by bridging the gap between the endopeptidase and aminopeptidase activities of the food vacuole. Two reversible cathepsin C inhibitors potently inhibited both recombinant and native DPAP1, thereby validating the use of recombinant DPAP1 for future inhibitor discovery and characterization.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
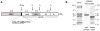
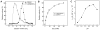

Similar articles
-
A Plasmodium falciparum dipeptidyl aminopeptidase I participates in vacuolar hemoglobin degradation.J Biol Chem. 2004 Oct 8;279(41):43000-7. doi: 10.1074/jbc.M408123200. Epub 2004 Aug 10. J Biol Chem. 2004. PMID: 15304495
-
Unnatural amino acids increase activity and specificity of synthetic substrates for human and malarial cathepsin C.Amino Acids. 2014 Apr;46(4):931-43. doi: 10.1007/s00726-013-1654-2. Epub 2014 Jan 1. Amino Acids. 2014. PMID: 24381006 Free PMC article.
-
Functional studies of Plasmodium falciparum dipeptidyl aminopeptidase I using small molecule inhibitors and active site probes.Chem Biol. 2010 Aug 27;17(8):808-19. doi: 10.1016/j.chembiol.2010.06.007. Chem Biol. 2010. PMID: 20797610 Free PMC article.
-
Falcipains and other cysteine proteases of malaria parasites.Adv Exp Med Biol. 2011;712:30-48. doi: 10.1007/978-1-4419-8414-2_3. Adv Exp Med Biol. 2011. PMID: 21660657 Review.
-
Development of Plasmodium falciparum protease inhibitors in the past decade (2002-2012).Curr Med Chem. 2013;20(25):3049-68. doi: 10.2174/0929867311320250003. Curr Med Chem. 2013. PMID: 23514416 Review.
Cited by
-
Using in Vitro Evolution and Whole Genome Analysis To Discover Next Generation Targets for Antimalarial Drug Discovery.ACS Infect Dis. 2018 Mar 9;4(3):301-314. doi: 10.1021/acsinfecdis.7b00276. Epub 2018 Feb 21. ACS Infect Dis. 2018. PMID: 29451780 Free PMC article. Review.
-
Identification of Plasmodium dipeptidyl aminopeptidase allosteric inhibitors by high throughput screening.PLoS One. 2019 Dec 18;14(12):e0226270. doi: 10.1371/journal.pone.0226270. eCollection 2019. PLoS One. 2019. PMID: 31851699 Free PMC article.
-
Heme Detoxification in the Malaria Parasite Plasmodium falciparum: A Time-Dependent Basal-Level Analysis.bioRxiv [Preprint]. 2025 Mar 6:2025.03.06.641703. doi: 10.1101/2025.03.06.641703. bioRxiv. 2025. PMID: 40093040 Free PMC article. Preprint.
-
Proteases as regulators of pathogenesis: examples from the Apicomplexa.Biochim Biophys Acta. 2012 Jan;1824(1):177-85. doi: 10.1016/j.bbapap.2011.06.002. Epub 2011 Jun 13. Biochim Biophys Acta. 2012. PMID: 21683169 Free PMC article. Review.
-
The antimalarial natural product symplostatin 4 is a nanomolar inhibitor of the food vacuole falcipains.Chem Biol. 2012 Dec 21;19(12):1546-55. doi: 10.1016/j.chembiol.2012.09.020. Chem Biol. 2012. PMID: 23261598 Free PMC article.
References
-
- Krugliak M, Zhang J, Ginsburg H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol Biochem Parasitol. 2002;119:249–56. - PubMed
-
- Goldberg DE. Hemoglobin degradation. Curr Top Microbiol Immunol. 2005;295:275–91. - PubMed
-
- Klemba M, Gluzman I, Goldberg DE. A Plasmodium falciparum dipeptidyl aminopeptidase I participates in vacuolar hemoglobin degradation. J Biol Chem. 2004;279:43000–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

