Identification of dispensable nucleotide sequence in 3' untranslated region of porcine reproductive and respiratory syndrome virus
- PMID: 20833212
- PMCID: PMC7114379
- DOI: 10.1016/j.virusres.2010.08.027
Identification of dispensable nucleotide sequence in 3' untranslated region of porcine reproductive and respiratory syndrome virus
Abstract
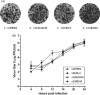
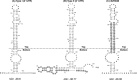
The 3' untranslated region (UTR) of porcine arterivirus genome plays a pivotal role for virus replication, yet the properties of 3' UTR remain largely undefined. We conducted site-directed mutagenesis to the 3' UTR of the type II porcine reproductive and respiratory syndrome virus (PRRSV). Serial deletions of the 3' UTR showed that at least 40 nucleotides immediately following the ORF7 stop codon were dispensable for the viability of PRRSV in cultured cells. We then constructed a chimeric PRRSV cDNA clone using type II PRRSV as the backbone containing the 3' UTR from the type I PRRSV. The chimeric virus was viable and shared similar properties with the parental virus. Our results provided the first description of the 40nt dispensable region in type I PRRSV 3' UTR, and further predicted structure demonstrated that the high-order structure of 3' UTR might play significant roles in its function.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures





References
-
- Beerens N., Snijder E.J. RNA signals in the 3′ terminus of the genome of Equine arteritis virus are required for viral RNA synthesis. J. Gen. Virol. 2006;87:1977–1983. - PubMed
-
- Chang K.S., Luo G. The polypyrimidine tract-binding protein (PTB) is required for efficient replication of hepatitis C virus (HCV) RNA. Virus Res. 2006;115:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

