Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques
- PMID: 20833230
- PMCID: PMC2987542
- DOI: 10.1016/j.neuroscience.2010.08.059
Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques
Abstract
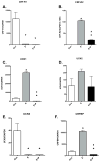
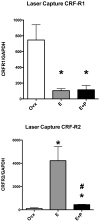
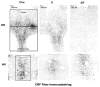
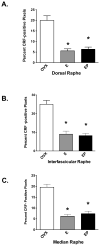
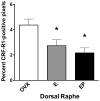
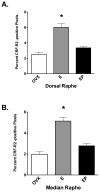
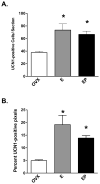
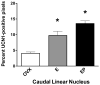
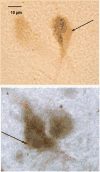
A significant number of postmenopausal women report increased anxiety and vulnerability to stress, which has been linked to decreased secretion of ovarian steroids. Communication between the serotonin system and the corticotropin releasing factor (CRF) system determines stress sensitivity or resilience. This study examines the effects of the ovarian steroids, estradiol (E) and progesterone (P) on the CRF system components that impact serotonin neurons in the midbrain of nonhuman primates. Ovariectomized rhesus macaques were treated with placebo, E alone for 1 month, or E supplemented with P for the last 2 weeks. Quantitative (q)RT-PCR and immunocytochemistry were employed. E±P treatment decreased CRF-R1 and increased CRF-R2 gene expression in hemi-midbrain blocks and in laser captured serotonin neurons. Also in hemi-midbrains, E treatment increased urocortin 1 (UCN1) and CRFBP gene expression, but supplemental P treatment reversed these effects. E±P decreased CRF fiber density in the dorsal, interfascicular and median raphe nuclei and decreased CRF-R1 immunostaining in the dorsal raphe. E increased CRF-R2 immunostaining in the dorsal and median raphe. E±P increased UCN1 immunostaining in the cell bodies and increased UCN1 fiber density in the caudal linear nucleus. Estrogen receptor beta (ERβ), but not ERα was detected in the nucleus of UCN1-positive neurons. While the mechanism of ovarian hormone regulation of the midbrain CRF system requires further investigation, these studies clearly demonstrate another pathway by which ovarian hormones may have positive effects on anxiety and mood regulation.
Copyright © 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Effects of citalopram on serotonin and CRF systems in the midbrain of primates with differences in stress sensitivity.J Chem Neuroanat. 2011 Jul;41(4):200-18. doi: 10.1016/j.jchemneu.2011.05.010. Epub 2011 Jun 6. J Chem Neuroanat. 2011. PMID: 21683135 Free PMC article. Review.
-
The effect of short moderate stress on the midbrain corticotropin-releasing factor system in a macaque model of functional hypothalamic amenorrhea.Fertil Steril. 2013 Oct;100(4):1111-21. doi: 10.1016/j.fertnstert.2013.05.052. Epub 2013 Jul 10. Fertil Steril. 2013. PMID: 23849846 Free PMC article.
-
Unique responses of midbrain CART neurons in macaques to ovarian steroids.Brain Res. 2008 Aug 28;1227:76-88. doi: 10.1016/j.brainres.2008.05.078. Epub 2008 Jun 10. Brain Res. 2008. PMID: 18598674 Free PMC article.
-
Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorrhea.Neuroendocrinology. 2010;92(4):224-34. doi: 10.1159/000319257. Epub 2010 Aug 12. Neuroendocrinology. 2010. PMID: 20714124 Free PMC article.
-
Protective actions of ovarian hormones in the serotonin system of macaques.Front Neuroendocrinol. 2009 Jul;30(2):212-38. doi: 10.1016/j.yfrne.2009.04.003. Epub 2009 Apr 24. Front Neuroendocrinol. 2009. PMID: 19394356 Free PMC article. Review.
Cited by
-
Effects of citalopram on serotonin and CRF systems in the midbrain of primates with differences in stress sensitivity.J Chem Neuroanat. 2011 Jul;41(4):200-18. doi: 10.1016/j.jchemneu.2011.05.010. Epub 2011 Jun 6. J Chem Neuroanat. 2011. PMID: 21683135 Free PMC article. Review.
-
The relation of developmental changes in brain serotonin transporter (5HTT) and 5HT1A receptor binding to emotional behavior in female rhesus monkeys: effects of social status and 5HTT genotype.Neuroscience. 2013 Jan 3;228:83-100. doi: 10.1016/j.neuroscience.2012.10.016. Epub 2012 Oct 16. Neuroscience. 2013. PMID: 23079633 Free PMC article.
-
Reproductive steroid receptors and actions in the locus coeruleus of male macaques: Part of an aggression circuit?Prog Neuropsychopharmacol Biol Psychiatry. 2016 Nov 3;71:210-22. doi: 10.1016/j.pnpbp.2016.04.002. Epub 2016 Apr 12. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 27083854 Free PMC article. Clinical Trial.
-
The effect of long-term ovariectomy on midbrain stress systems in free ranging macaques.Brain Res. 2012 Dec 7;1488:24-37. doi: 10.1016/j.brainres.2012.09.035. Epub 2012 Oct 1. Brain Res. 2012. PMID: 23036275 Free PMC article.
-
Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis.Psychoneuroendocrinology. 2016 Jan;63:178-90. doi: 10.1016/j.psyneuen.2015.09.024. Epub 2015 Sep 30. Psychoneuroendocrinology. 2016. PMID: 26454419 Free PMC article.
References
-
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. - PubMed
-
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. - PubMed
-
- Austin MC, Rice PM, Mann JJ, Arango V. Localization of corticotropin-releasing hormone in the human locus coeruleus and pedunculopontine tegmental nucleus: an immunocytochemical and in situ hybridization study. Neuroscience. 1995;64:713–727. - PubMed
-
- Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. - PubMed
-
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32-HD007133/HD/NICHD NIH HHS/United States
- P51 RR000163/RR/NCRR NIH HHS/United States
- R01 MH062677/MH/NIMH NIH HHS/United States
- RR00163/RR/NCRR NIH HHS/United States
- U54 HD 18185/HD/NICHD NIH HHS/United States
- T32 DK007680/DK/NIDDK NIH HHS/United States
- MH62677/MH/NIMH NIH HHS/United States
- K01 RR000163/RR/NCRR NIH HHS/United States
- T32 HD007133/HD/NICHD NIH HHS/United States
- P30 NS061800/NS/NINDS NIH HHS/United States
- U54 HD018185/HD/NICHD NIH HHS/United States
- P30 HD018185/HD/NICHD NIH HHS/United States
- T32-DK07680/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

