Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia
- PMID: 20833242
- PMCID: PMC3010320
- DOI: 10.1016/j.ijdevneu.2010.08.007
Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia
Abstract
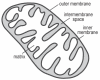
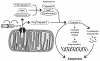
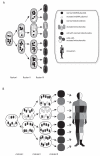
Bipolar disorder (BPD) and schizophrenia (SZ) are severe psychiatric illnesses with a combined prevalence of 4%. A disturbance of energy metabolism is frequently observed in these disorders. Several pieces of evidence point to an underlying dysfunction of mitochondria: (i) decreased mitochondrial respiration; (ii) changes in mitochondrial morphology; (iii) increases in mitochondrial DNA (mtDNA) polymorphisms and in levels of mtDNA mutations; (iv) downregulation of nuclear mRNA molecules and proteins involved in mitochondrial respiration; (v) decreased high-energy phosphates and decreased pH in the brain; and (vi) psychotic and affective symptoms, and cognitive decline in mitochondrial disorders. Furthermore, transgenic mice with mutated mitochondrial DNA polymerase show mood disorder-like phenotypes. In this review, we will discuss the genetic and physiological components of mitochondria and the evidence for mitochondrial abnormalities in BPD and SZ. We will furthermore describe the role of mitochondria during brain development and the effect of current drugs for mental illness on mitochondrial function. Understanding the role of mitochondria, both developmentally as well as in the ailing brain, is of critical importance to elucidate pathophysiological mechanisms in psychiatric disorders.
Copyright © 2010 ISDN. Published by Elsevier Ltd. All rights reserved.
Figures
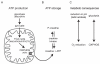

References
-
- Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, Young TA, Bullard J, Yokoe H, Webster MJ, Knable MB, Brockman JA. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. - PubMed
-
- Amar S, Shamir A, Ovadia O, Blanaru M, Reshef A, Kremer I, Rietschel M, Schulze TG, Maier W, Belmaker RH, Ebstein RP, Agam G, Mishmar D. Mitochondrial DNA HV lineage increases the susceptibility to schizophrenia among Israeli Arabs. Schizophr Res. 2007;94:354–358. - PubMed
-
- Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. - PubMed
-
- Arinami T. Analyses of the associations between the genes of 22q11 deletion syndrome and schizophrenia. J Hum Genet. 2006;51:1037–1045. - PubMed

