A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells
- PMID: 20833374
- PMCID: PMC2991069
- DOI: 10.1016/j.chom.2010.08.002
A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells
Abstract
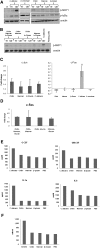
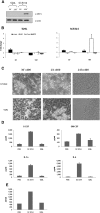
Discriminating between commensal and pathogenic states of opportunistic pathogens is critical for host mucosal defense and homeostasis. The opportunistic human fungal pathogen Candida albicans is also a constituent of the normal oral flora and grows either as yeasts or hyphae. We demonstrate that oral epithelial cells orchestrate an innate response to C. albicans via NF-κB and a biphasic MAPK response. Activation of NF-κB and the first MAPK phase, constituting c-Jun activation, is independent of morphology and due to fungal cell wall recognition. Activation of the second MAPK phase, constituting MKP1 and c-Fos activation, is dependent upon hypha formation and fungal burdens and correlates with proinflammatory responses. Such biphasic response may allow epithelial tissues to remain quiescent under low fungal burdens while responding specifically and strongly to damage-inducing hyphae when burdens increase. MAPK/MKP1/c-Fos activation may represent a "danger response" pathway that is critical for identifying and responding to the pathogenic switch of commensal microbes.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Epithelial sensing of fungal invasion.Cell Host Microbe. 2010 Sep 16;8(3):219-20. doi: 10.1016/j.chom.2010.08.008. Cell Host Microbe. 2010. PMID: 20833371
-
One-two blow alerts immune system.Nat Rev Immunol. 2010 Nov;10(11):752. doi: 10.1038/nri2882. Nat Rev Immunol. 2010. PMID: 21080615 No abstract available.
References
-
- Abreu M.T., Fukata M., Arditi M. TLR signaling in the gut in health and disease. J. Immunol. 2005;174:4453–4460. - PubMed
-
- Agrawal S., Agrawal A., Doughty B., Gerwitz A., Blenis J., Van Dyke T., Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 2003;171:4984–4989. - PubMed
-
- Braun B.R., Johnson A.D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. - PubMed
-
- Brondello J.M., Pouysségur J., McKenzie F.R. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

