In vivo mechanisms of vaccine-induced protection against HPV infection
- PMID: 20833377
- PMCID: PMC2939057
- DOI: 10.1016/j.chom.2010.08.003
In vivo mechanisms of vaccine-induced protection against HPV infection
Abstract
Using a human papillomavirus (HPV) cervicovaginal murine challenge model, we microscopically examined the in vivo mechanisms of L1 virus-like particle (VLP) and L2 vaccine-induced inhibition of infection. In vivo HPV infection requires an initial association with the acellular basement membrane (BM) to induce conformational changes in the virion that permit its association with the keratinocyte cell surface. By passive transfer of immune serum, we determined that anti-L1 antibodies can interfere with infection at two stages. Similarly to active VLP immunization, transfer of high L1 antibody concentrations prevented BM binding. However, in the presence of low concentrations of anti-L1, virions associated with the BM, but to the epithelial cell surface was not detected. Regardless of the concentration, L2 vaccine-induced antibodies allow BM association but prevent association with the cell surface. Thus, we have revealed distinct mechanisms of vaccine-induced inhibition of virus infection in vivo.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

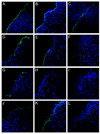
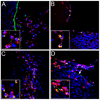


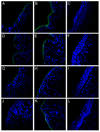
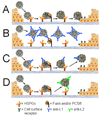
Comment in
-
Stopping HPVs dead in their tracts.Cell Host Microbe. 2010 Sep 16;8(3):221-2. doi: 10.1016/j.chom.2010.08.010. Cell Host Microbe. 2010. PMID: 20833372
References
-
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, et al. The Impact of Quadrivalent Human Papillomavirus (HPV; Types 6, 11, 16, and 18) L1 Virus-Like Particle Vaccine on Infection and Disease Due to Oncogenic Nonvaccine HPV Types in Generally HPV-Naive Women Aged 16–26 Years. The Journal of Infectious Diseases. 2009;199:926–935. - PubMed
-
- Buck CB, Thompson CD. Production of papillomavirus-based gene transfer vectors. Curr Protoc Cell Biol. 2007;Chapter 26(Unit 26 21) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

