Contribution of lysine 11-linked ubiquitination to MIR2-mediated major histocompatibility complex class I internalization
- PMID: 20833710
- PMCID: PMC2975155
- DOI: 10.1074/jbc.M110.112763
Contribution of lysine 11-linked ubiquitination to MIR2-mediated major histocompatibility complex class I internalization
Abstract
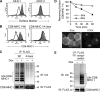
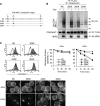
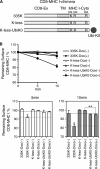
The polyubiquitin chain is generated by the sequential addition of ubiquitin moieties to target molecules, a reaction between specific lysine residues that is catalyzed by E3 ubiquitin ligase. The Lys(48)-linked and Lys(63)-linked polyubiquitin chains are well established inducers of proteasome-dependent degradation and signal transduction, respectively. The concept has recently emerged that polyubiquitin chain-mediated regulation is even more complex because various types of atypical polyubiquitin chains have been discovered in vivo. Here, we demonstrate that a novel complex ubiquitin chain functions as an internalization signal for major histocompatibility complex class I (MHC I) membrane proteins in vivo. Using a tetracycline-inducible expression system and quantitative mass spectrometry, we show that the polyubiquitin chain generated by the viral E3 ubiquitin ligase of Kaposi sarcoma-associated herpesvirus, MIR2, is a Lys(11) and Lys(63) mixed-linkage chain. This novel ubiquitin chain can function as an internalization signal for MHC I through its association with epsin1, an adaptor molecule containing ubiquitin-interacting motifs.
Figures



References
-
- Raiborg C., Stenmark H. (2009) Nature 458, 445–452 - PubMed
-
- Shtiegman K., Kochupurakkal B. S., Zwang Y., Pines G., Starr A., Vexler A., Citri A., Katz M., Lavi S., Ben-Basat Y., Benjamin S., Corso S., Gan J., Yosef R. B., Giordano S., Yarden Y. (2007) Oncogene 26, 6968–6978 - PubMed
-
- Gómez-Martín D., Díaz-Zamudio M., Alcocer-Varela J. (2008) Autoimmun. Rev. 7, 284–290 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

