Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial
- PMID: 20833738
- PMCID: PMC3002764
- DOI: 10.1136/ard.2010.131995
Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial
Abstract
Background: Long-term immunosuppressive treatment does not efficiently prevent relapses of lupus nephritis (LN). This investigator-initiated randomised trial tested whether mycophenolate mofetil (MMF) was superior to azathioprine (AZA) as maintenance treatment.
Methods: A total of 105 patients with lupus with proliferative LN were included. All received three daily intravenous pulses of 750 mg methylprednisolone, followed by oral glucocorticoids and six fortnightly cyclophosphamide intravenous pulses of 500 mg. Based on randomisation performed at baseline, AZA (target dose: 2 mg/kg/day) or MMF (target dose: 2 g/day) was given at week 12. Analyses were by intent to treat. Time to renal flare was the primary end point. Mean (SD) follow-up of the intent-to-treat population was 48 (14) months.
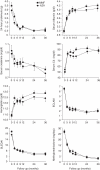
Results: The baseline clinical, biological and pathological characteristics of patients allocated to AZA or MMF did not differ. Renal flares were observed in 13 (25%) AZA-treated and 10 (19%) MMF-treated patients. Time to renal flare, to severe systemic flare, to benign flare and to renal remission did not statistically differ. Over a 3-year period, 24 h proteinuria, serum creatinine, serum albumin, serum C3, haemoglobin and global disease activity scores improved similarly in both groups. Doubling of serum creatinine occurred in four AZA-treated and three MMF-treated patients. Adverse events did not differ between the groups except for haematological cytopenias, which were statistically more frequent in the AZA group (p=0.03) but led only one patient to drop out.
Conclusions: Fewer renal flares were observed in patients receiving MMF but the difference did not reach statistical significance.
Conflict of interest statement
Figures


Comment in
-
A decade of mycophenolate mofetil for lupus nephritis: is the glass half-empty or half-full?Ann Rheum Dis. 2010 Dec;69(12):2059-61. doi: 10.1136/ard.2010.139683. Ann Rheum Dis. 2010. PMID: 21097656 Free PMC article. No abstract available.
References
-
- Cameron JS. Lupus nephritis. J Am Soc Nephrol 1999;10:413–24 - PubMed
-
- Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308 - PubMed
-
- Austin HA, 3rd, Klippel JH, Balow JE, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 1986;314:614–19 - PubMed
-
- Boumpas DT, Austin HA, 3rd, Vaughn EM, et al. Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 1992;340:741–5 - PubMed
-
- Gourley MF, Austin HA, 3rd, Scott D, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med 1996;125:549–57 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

