Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study
- PMID: 20833740
- PMCID: PMC2938899
- DOI: 10.1136/bmj.c4372
Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study
Abstract
Objectives: To determine the accuracy and acceptability to patients of non-endoscopic screening for Barrett's oesophagus, using an ingestible oesophageal sampling device (Cytosponge) coupled with immunocytochemisty for trefoil factor 3.
Design: Prospective cohort study.
Setting: 12 UK general practices, with gastroscopies carried out in one hospital endoscopy unit.
Participants: 504 of 2696 eligible patients (18.7%) aged 50 to 70 years with a previous prescription for an acid suppressant (H(2) receptor antagonist or proton pump inhibitor) for more than three months in the past five years.
Main outcome measures: Sensitivity and specificity estimates for detecting Barrett's oesophagus compared with gastroscopy as the ideal method, and patient anxiety (short form Spielberger state trait anxiety inventory, impact of events scale) and acceptability (visual analogue scale) of the test.
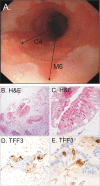
Results: 501 of 504 (99%) participants (median age 62, male to female ratio 1:1.2) successfully swallowed the Cytosponge. No serious adverse events occurred. In total, 3.0% (15/501) had an endoscopic diagnosis of Barrett's oesophagus (≥1 cm circumferential length, median circumferential and maximal length of 2 cm and 5 cm, respectively) with intestinal metaplasia. Compared with gastroscopy the sensitivity and specificity of the test was 73.3% (95% confidence interval 44.9% to 92.2%) and 93.8% (91.3% to 95.8%) for 1 cm or more circumferential length and 90.0% (55.5% to 99.7%) and 93.5% (90.9% to 95.5%) for clinically relevant segments of 2 cm or more. Most participants (355/496, 82%, 95% confidence interval 78.9% to 85.1%) reported low levels of anxiety before the test, and scores remained within normal limits at follow-up. Less than 4.5% (2.8% to 6.1%) of participants reported psychological distress a week after the procedure.
Conclusions: The performance of the Cytosponge test was promising and the procedure was well tolerated. These data bring screening for Barrett's oesophagus into the realm of possibility. Further evaluation is recommended.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures



Comment in
-
Non-endoscopic screening for Barrett's oesophagus.BMJ. 2010 Sep 10;341:c4667. doi: 10.1136/bmj.c4667. BMJ. 2010. PMID: 20833744 No abstract available.
-
Cytosponge for Barrett's esophagus screening: when smart science matches simplicity.Gastroenterology. 2011 Aug;141(2):766-8; discussion 768. doi: 10.1053/j.gastro.2011.06.012. Epub 2011 Jun 23. Gastroenterology. 2011. PMID: 21704039 No abstract available.
References
-
- Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet 2009;373:850-61. - PubMed
-
- Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol 2008;168:237-49. - PubMed
-
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. - PubMed
-
- Shaheen NJ, Sharma P, Overholt BF, Lightdale CJ, Wolfsen HC, Sampliner RE, et al. A randomized, multicenter, sham-controlled trial of radiofrequency ablation (RFA) for subjects with Barrett’s esophagus (BE) containing dysplasia: interim results of the AIM Dysplasia Trial. Gastroenterology 2008;134:750-8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
