Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells
- PMID: 20833776
- PMCID: PMC3006268
- DOI: 10.1152/ajplung.00233.2010
Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells
Abstract
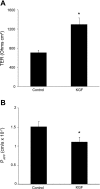
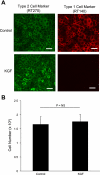
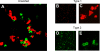
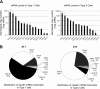
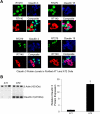
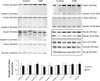
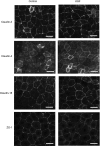
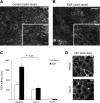
Keratinocyte growth factor (KGF) has efficacy in several experimental models of lung injury; however, the mechanisms underlying KGF's protective effect remain incompletely understood. This study was undertaken to determine whether KGF augments barrier function in primary rat alveolar epithelial cells grown in culture, specifically whether KGF alters tight junction function via claudin expression. KGF significantly increased alveolar epithelial barrier function in culture as assessed by transepithelial electrical resistance (TER) and paracellular permeability. Fluorescence-activated cell sorting of freshly isolated type 1 (AT1) and type 2 (AT2) cells followed by quantitative real-time RT-PCR revealed that more than 97% of claudin mRNA transcripts in these cells were for claudins-3, -4, and -18. Using cultured AT2 cells, we then examined the effect of KGF on the protein levels of the claudins with the highest mRNA levels: -3, -4, -5, -7, -12, -15, and -18. KGF did not alter the levels of any of the claudins tested, nor of zona occludens-1 (ZO-1) or occludin. Moreover, localization of claudins-3, -4, -18, and ZO-1 was unchanged. KGF did induce a marked increase in the apical perijunctional F-actin ring. Actin depolymerization with cytochalasin D blocked the KGF-mediated increase in TER without significantly changing TER in control cells. Together, these data support a novel mechanism by which KGF enhances alveolar barrier function, modulation of the actin cytoskeleton. In addition, these data demonstrate the complete claudin expression profile for AT1 and AT2 cells and indicate that claudins-3, -4, and -18 are the primary claudins expressed in these cell types.
Figures
Comment in
-
Keratinocyte growth factor improves alveolar barrier function: keeping claudins in line.Am J Physiol Lung Cell Mol Physiol. 2010 Dec;299(6):L721-3. doi: 10.1152/ajplung.00365.2010. Epub 2010 Oct 15. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20952495 Free PMC article. No abstract available.
Similar articles
-
Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis.Am J Physiol Lung Cell Mol Physiol. 2012 Jan 15;302(2):L193-205. doi: 10.1152/ajplung.00349.2010. Epub 2011 Oct 14. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22003091
-
Knockout mice reveal key roles for claudin 18 in alveolar barrier properties and fluid homeostasis.Am J Respir Cell Mol Biol. 2014 Aug;51(2):210-22. doi: 10.1165/rcmb.2013-0353OC. Am J Respir Cell Mol Biol. 2014. PMID: 24588076 Free PMC article.
-
Heterogeneity of claudin expression by alveolar epithelial cells.Am J Respir Cell Mol Biol. 2003 Jul;29(1):62-70. doi: 10.1165/rcmb.2002-0180OC. Epub 2003 Jan 23. Am J Respir Cell Mol Biol. 2003. PMID: 12600828
-
Ruffles and spikes: Control of tight junction morphology and permeability by claudins.Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183339. doi: 10.1016/j.bbamem.2020.183339. Epub 2020 May 7. Biochim Biophys Acta Biomembr. 2020. PMID: 32389670 Free PMC article. Review.
-
Claudins and alveolar epithelial barrier function in the lung.Ann N Y Acad Sci. 2012 Jun;1257:175-83. doi: 10.1111/j.1749-6632.2012.06533.x. Ann N Y Acad Sci. 2012. PMID: 22671604 Free PMC article. Review.
Cited by
-
A Monoclonal Human Alveolar Epithelial Cell Line ("Arlo") with Pronounced Barrier Function for Studying Drug Permeability and Viral Infections.Adv Sci (Weinh). 2023 Mar;10(8):e2207301. doi: 10.1002/advs.202207301. Epub 2023 Feb 7. Adv Sci (Weinh). 2023. PMID: 36748276 Free PMC article.
-
Fatty acid synthase downregulation contributes to acute lung injury in murine diet-induced obesity.JCI Insight. 2019 Jul 9;5(15):e127823. doi: 10.1172/jci.insight.127823. JCI Insight. 2019. PMID: 31287803 Free PMC article.
-
New insights into the mechanisms of pulmonary edema in acute lung injury.Ann Transl Med. 2018 Jan;6(2):32. doi: 10.21037/atm.2017.12.18. Ann Transl Med. 2018. PMID: 29430449 Free PMC article. Review.
-
Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs.Tissue Barriers. 2018;6(4):1-13. doi: 10.1080/21688370.2018.1540904. Epub 2018 Nov 8. Tissue Barriers. 2018. PMID: 30409076 Free PMC article.
-
Palifermin for the protection and regeneration of epithelial tissues following injury: new findings in basic research and pre-clinical models.J Cell Mol Med. 2013 Sep;17(9):1065-87. doi: 10.1111/jcmm.12091. J Cell Mol Med. 2013. PMID: 24151975 Free PMC article. Review.
References
-
- Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 276: L825–L834, 1999 - PubMed
-
- Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30: 35–42, 1974 - PubMed
-
- Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002 - PubMed
-
- Baba Y, Yazawa T, Kanegae Y, Sakamoto S, Saito I, Morimura N, Goto T, Yamada Y, Kurahashi K. Keratinocyte growth factor gene transduction ameliorates acute lung injury and mortality in mice. Hum Gene Ther 18: 130–141, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

