Proteomic analysis of Neorickettsia sennetsu surface-exposed proteins and porin activity of the major surface protein P51
- PMID: 20833807
- PMCID: PMC2976439
- DOI: 10.1128/JB.00632-10
Proteomic analysis of Neorickettsia sennetsu surface-exposed proteins and porin activity of the major surface protein P51
Abstract
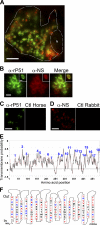
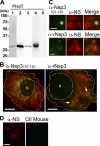
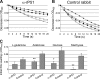
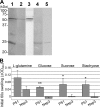
Neorickettsia sennetsu is an obligate intracellular bacterium of monocytes and macrophages and is the etiologic agent of human Sennetsu neorickettsiosis. Neorickettsia proteins expressed in mammalian host cells, including the surface proteins of Neorickettsia spp., have not been defined. In this paper, we isolated surface-exposed proteins from N. sennetsu by biotin surface labeling followed by streptavidin-affinity chromatography. Forty-two of the total of 936 (4.5%) N. sennetsu open reading frames (ORFs) were detected by liquid chromatography-tandem mass spectrometry (LC/MS/MS), including six hypothetical proteins. Among the major proteins identified were the two major β-barrel proteins: the 51-kDa antigen (P51) and Neorickettsia surface protein 3 (Nsp3). Immunofluorescence labeling not only confirmed surface exposure of these proteins but also showed rosary-like circumferential labeling with anti-P51 for the majority of bacteria and polar to diffuse punctate labeling with anti-Nsp3 for a minority of bacteria. We found that the isolated outer membrane of N. sennetsu had porin activity, as measured by a proteoliposome swelling assay. This activity allowed the diffusion of L-glutamine, the monosaccharides arabinose and glucose, and the tetrasaccharide stachyose, which could be inhibited with anti-P51 antibody. We purified native P51 and Nsp3 under nondenaturing conditions. When reconstituted into proteoliposomes, purified P51, but not Nsp3, exhibited prominent porin activity. This the first proteomic study of a Neorickettsia sp. showing new sets of proteins evolved as major surface proteins for Neorickettsia and the first identification of a porin for the genus Neorickettsia.
Figures
Similar articles
-
Neorickettsia risticii surface-exposed proteins: proteomics identification, recognition by naturally-infected horses, and strain variations.Vet Res. 2011 Jun 2;42(1):71. doi: 10.1186/1297-9716-42-71. Vet Res. 2011. PMID: 21635728 Free PMC article.
-
Analysis of p51, groESL, and the major antigen P51 in various species of Neorickettsia, an obligatory intracellular bacterium that infects trematodes and mammals.J Clin Microbiol. 2004 Aug;42(8):3823-6. doi: 10.1128/JCM.42.8.3823-3826.2004. J Clin Microbiol. 2004. PMID: 15297539 Free PMC article.
-
Porin activity of Anaplasma phagocytophilum outer membrane fraction and purified P44.J Bacteriol. 2007 Mar;189(5):1998-2006. doi: 10.1128/JB.01548-06. Epub 2006 Dec 15. J Bacteriol. 2007. PMID: 17172334 Free PMC article.
-
Pore-forming activity of Coxiella burnetii outer membrane protein oligomer comprised of 29.5- and 31-kDa polypeptides. Inhibition of porin activity by monoclonal antibodies 4E8 and 4D6.Ann N Y Acad Sci. 1996 Jul 23;791:378-401. doi: 10.1111/j.1749-6632.1996.tb53545.x. Ann N Y Acad Sci. 1996. PMID: 8784519
-
Surface antigens of the syphilis spirochete and their potential as virulence determinants.Emerg Infect Dis. 1997 Jan-Mar;3(1):11-20. doi: 10.3201/eid0301.970102. Emerg Infect Dis. 1997. PMID: 9126440 Free PMC article. Review.
Cited by
-
Secretome of obligate intracellular Rickettsia.FEMS Microbiol Rev. 2015 Jan;39(1):47-80. doi: 10.1111/1574-6976.12084. Epub 2014 Dec 4. FEMS Microbiol Rev. 2015. PMID: 25168200 Free PMC article. Review.
-
Surface proteome analysis and characterization of surface cell antigen (Sca) or autotransporter family of Rickettsia typhi.PLoS Pathog. 2012;8(8):e1002856. doi: 10.1371/journal.ppat.1002856. Epub 2012 Aug 9. PLoS Pathog. 2012. PMID: 22912578 Free PMC article.
-
Analysis of complete genome sequence and major surface antigens of Neorickettsia helminthoeca, causative agent of salmon poisoning disease.Microb Biotechnol. 2017 Jul;10(4):933-957. doi: 10.1111/1751-7915.12731. Epub 2017 Jun 6. Microb Biotechnol. 2017. PMID: 28585301 Free PMC article.
-
Genomes of Fasciola hepatica from the Americas Reveal Colonization with Neorickettsia Endobacteria Related to the Agents of Potomac Horse and Human Sennetsu Fevers.PLoS Genet. 2017 Jan 6;13(1):e1006537. doi: 10.1371/journal.pgen.1006537. eCollection 2017 Jan. PLoS Genet. 2017. PMID: 28060841 Free PMC article.
-
Factor H Is Bound by Outer Membrane-Displayed Carbohydrate Metabolism Enzymes of Extraintestinal Pathogenic Escherichia coli and Contributes to Opsonophagocytosis Resistance in Bacteria.Front Cell Infect Microbiol. 2021 Jan 25;10:592906. doi: 10.3389/fcimb.2020.592906. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33569353 Free PMC article.
References
-
- Baldermann, C., and H. Engelhardt. 2000. Expression, two-dimensional crystallization, and three-dimensional reconstruction of the beta8 outer membrane protein Omp21 from Comamonas acidovorans. J. Struct. Biol. 131:96-107. - PubMed
-
- Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

