Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the DCCT/EDIC study
- PMID: 20833868
- PMCID: PMC2992204
- DOI: 10.2337/dc10-0616
Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the DCCT/EDIC study
Abstract
Objective: To describe the sensitivity, specificity, positive predictive value, and negative predictive value of vibration perception threshold (VPT) testing in subjects with type 1 diabetes relative to gold standard assessments of peripheral neuropathy.
Research design and methods: VPT was determined in 1,177 adults with type 1 diabetes 13-14 years after participating in a study of intensive (INT) versus conventional (CONV) diabetes treatment. Abnormal VPT was defined by values exceeding 2.5 SD above age-specific normal values. Signs and symptoms of peripheral neuropathy were assessed and electrodiagnostic studies were performed to establish definite clinical neuropathy, abnormal nerve conduction, and confirmed clinical neuropathy (the presence of both definite clinical neuropathy and abnormal nerve conduction).
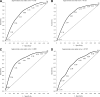
Results: Thirty-seven percent of subjects had definite clinical neuropathy, 61% had abnormal nerve conduction, and 30% had confirmed clinical neuropathy. Abnormal VPT was more common among former CONV than among INT subjects (64 vs. 57%, P < 0.05) and was associated with older age. VPT was a sensitive measure of confirmed clinical neuropathy (87%) and of definite clinical neuropathy (80%) and a specific measure of abnormal nerve conduction (62%). Higher VPT cut points improved test sensitivity and lower cut points improved specificity. Areas under the receiver operating characteristic curves ranged from 0.71-0.83 and were higher for older than for younger subjects and highest for those with confirmed clinical neuropathy.
Conclusions: VPT was a sensitive measure of peripheral neuropathy. Future researchers may choose to select VPT cut points for defining abnormality based on the population studied and clinical outcome of interest.
Trial registration: ClinicalTrials.gov NCT00360893.
Figures


References
-
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–986 - PubMed
-
- The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group. Ann Intern Med 1995;122:561–568 - PubMed
-
- Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol 1995;38:869–880 - PubMed
-
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA: A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 - PubMed

