Schizosaccharomyces pombe calmodulin, Cam1, plays a crucial role in sporulation by recruiting and stabilizing the spindle pole body components responsible for assembly of the forespore membrane
- PMID: 20833892
- PMCID: PMC3008279
- DOI: 10.1128/EC.00022-10
Schizosaccharomyces pombe calmodulin, Cam1, plays a crucial role in sporulation by recruiting and stabilizing the spindle pole body components responsible for assembly of the forespore membrane
Abstract
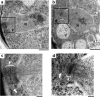
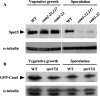
Calmodulin in Schizosaccharomyces pombe is encoded by the cam1(+) gene, which is indispensable for both vegetative growth and sporulation. Here, we report how Cam1 functions in spore formation. We found that Cam1 preferentially localized to the spindle pole body (SPB) during meiosis and sporulation. Formation of the forespore membrane, a precursor of the plasma membrane in spores, was blocked in a missense cam1 mutant, which was viable but unable to sporulate. Three SPB proteins necessary for the onset of forespore membrane formation, Spo2, Spo13, and Spo15, were unable to localize to the SPB in the cam1 mutant although five core SPB components that were tested were present. Recruitment of Spo2 and Spo13 is known to require the presence of Spo15 in the SPB. Notably, Spo15 was unstable in the cam1 mutant, and as a result, SPB localization of Spo2 and Spo13 was lost. Overexpression of Spo15 partially alleviated the sporulation defect in the cam1 mutant. These results indicate that calmodulin plays an essential role in forespore membrane formation by stably maintaining Spo15, and thus Spo2 and Spo13, at the SPB in meiotic cells.
Figures





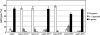
References
-
- Bennett M. K., Calakos N., Scheller R. H. 1992. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257:255–259 - PubMed
-
- Chikashige Y., Ding D. Q., Funabiki H., Mashiko S., Yanagida M., Hiraoka Y. 1994. Telomere-led premeiotic chromosome movement in fission yeast. Science 264:270–273 - PubMed
-
- Cyert M. S. 2001. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu. Rev. Genet. 35:647–672 - PubMed
-
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. 1986. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell 47:423–431 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

