Transcription profiling in human platelets reveals LRRFIP1 as a novel protein regulating platelet function
- PMID: 20833976
- PMCID: PMC2996120
- DOI: 10.1182/blood-2010-04-280925
Transcription profiling in human platelets reveals LRRFIP1 as a novel protein regulating platelet function
Abstract
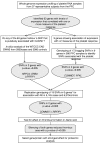
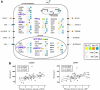
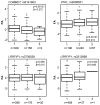
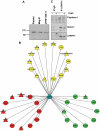
Within the healthy population, there is substantial, heritable, and interindividual variability in the platelet response. We explored whether a proportion of this variability could be accounted for by interindividual variation in gene expression. Through a correlative analysis of genome-wide platelet RNA expression data from 37 subjects representing the normal range of platelet responsiveness within a cohort of 500 subjects, we identified 63 genes in which transcript levels correlated with variation in the platelet response to adenosine diphosphate and/or the collagen-mimetic peptide, cross-linked collagen-related peptide. Many of these encode proteins with no reported function in platelets. An association study of 6 of the 63 genes in 4235 cases and 6379 controls showed a putative association with myocardial infarction for COMMD7 (COMM domain-containing protein 7) and a major deviation from the null hypo thesis for LRRFIP1 [leucine-rich repeat (in FLII) interacting protein 1]. Morpholino-based silencing in Danio rerio identified a modest role for commd7 and a significant effect for lrrfip1 as positive regulators of thrombus formation. Proteomic analysis of human platelet LRRFIP1-interacting proteins indicated that LRRFIP1 functions as a component of the platelet cytoskeleton, where it interacts with the actin-remodeling proteins Flightless-1 and Drebrin. Taken together, these data reveal novel proteins regulating the platelet response.
Figures


References
-
- Jones CI, Garner SF, Angenent W, et al. Mapping the platelet profile for functional genomic studies and demonstration of the effect size of the GP6 locus. J Thromb Haemost. 2007;5(8):1756–1765. - PubMed
-
- Panzer S, Hocker L, Koren D. Agonists-induced platelet activation varies considerably in healthy male individuals: studies by flow cytometry. Ann Hematol. 2006;85(2):121–125. - PubMed
-
- O'Donnell CJ, Larson MG, Feng D, et al. Genetic and environmental contributions to platelet aggregation: the Framingham Heart Study. Circulation. 2001;103(25):3051–3056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

