Differences in signalling by directly and indirectly binding ligands in bacterial chemotaxis
- PMID: 20834231
- PMCID: PMC2964171
- DOI: 10.1038/emboj.2010.224
Differences in signalling by directly and indirectly binding ligands in bacterial chemotaxis
Abstract
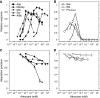
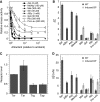
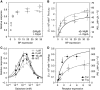
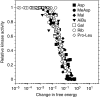
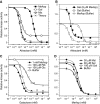
In chemotaxis of Escherichia coli and other bacteria, extracellular stimuli are perceived by transmembrane receptors that bind their ligands either directly, or indirectly through periplasmic-binding proteins (BPs). As BPs are also involved in ligand uptake, they provide a link between chemotaxis and nutrient utilization by cells. However, signalling by indirectly binding ligands remains much less understood than signalling by directly binding ligands. Here, we compared intracellular responses mediated by both types of ligands and developed a new mathematical model for signalling by indirectly binding ligands. We show that indirect binding allows cells to better control sensitivity to specific ligands in response to their nutrient environment and to coordinate chemotaxis with ligand transport, but at the cost of the dynamic range being much narrower than for directly binding ligands. We further demonstrate that signal integration by the chemosensory complexes does not depend on the type of ligand. Overall, our data suggest that the distinction between signalling by directly and indirectly binding ligands is more physiologically important than the traditional distinction between high- and low-abundance receptors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
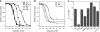
Comment in
-
Classifying chemoreceptors: quantity versus quality.EMBO J. 2010 Oct 20;29(20):3435-6. doi: 10.1038/emboj.2010.246. EMBO J. 2010. PMID: 20959858 Free PMC article.
References
-
- Adler J, Tso WW (1974) ‘Decision'-making in bacteria: chemotactic response of Escherichia coli to conflicting stimuli. Science 184: 1292–1294 - PubMed
-
- Anraku Y (1968) Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem 243: 3116–3122 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

