Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1
- PMID: 20835248
- PMCID: PMC3058254
- DOI: 10.1038/nm.2201
Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1
Abstract
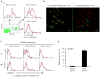
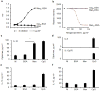
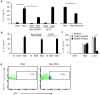
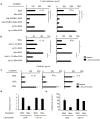
We propose that a C-type lectin receptor, SIGNR-1 (also called Cd209b), helps to condition dendritic cells (DCs) in the gastrointestinal lamina propria (LPDCs) for the induction of oral tolerance in a model of food-induced anaphylaxis. Oral delivery of BSA bearing 51 molecules of mannoside (Man(51)-BSA) substantially reduced the BSA-induced anaphylactic response. Man(51)-BSA selectively targeted LPDCs that expressed SIGNR1 and induced the expression of interleukin-10 (IL-10), but not IL-6 or IL-12 p70. We found the same effects in IL-10-GFP knock-in (tiger) mice treated with Man(51)-BSA. The Man(51)-BSA-SIGNR1 axis in LPDCs, both in vitro and in vivo, promoted the generation of CD4(+) type 1 regulatory T (Tr1)-like cells that expressed IL-10 and interferon-γ (IFN-γ), in a SIGNR-1- and IL-10-dependent manner, but not of CD4(+)CD25(+)Foxp3(+) regulatory T cells. The Tr1-like cells could transfer tolerance. These results suggest that sugar-modified antigens might be used to induce oral tolerance by targeting SIGNR1 and LPDCs.
Figures

Comment in
-
A sweet path toward tolerance in the gut.Nat Med. 2010 Oct;16(10):1076-7. doi: 10.1038/nm1010-1076. Nat Med. 2010. PMID: 20930744 No abstract available.
References
-
- Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. - PubMed
-
- Poulsen LK. In search of a new paradigm: mechanisms of sensitization and elicitation of food allergy. Allergy. 2005;60:549–58. - PubMed
-
- Finkelman FD. Anaphylaxis: Lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–15. - PubMed
-
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

