Clarke's column neurons as the focus of a corticospinal corollary circuit
- PMID: 20835249
- PMCID: PMC2947611
- DOI: 10.1038/nn.2637
Clarke's column neurons as the focus of a corticospinal corollary circuit
Abstract
Proprioceptive sensory signals inform the CNS of the consequences of motor acts, but effective motor planning involves internal neural systems capable of anticipating actual sensory feedback. Just where and how predictive systems exert their influence remains poorly understood. We explored the possibility that spinocerebellar neurons that convey proprioceptive sensory information also integrate information from cortical command systems. Analysis of the circuitry and physiology of identified dorsal spinocerebellar tract neurons in mouse spinal cord revealed distinct populations of Clarke's column neurons that received direct excitatory and/or indirect inhibitory inputs from descending corticospinal axons. The convergence of these descending inhibitory and excitatory inputs to Clarke's column neurons established local spinal circuits with the capacity to mark or modulate incoming proprioceptive input. Together, our genetic, anatomical and physiological results indicate that Clarke's column spinocerebellar neurons nucleate local spinal corollary circuits that are relevant to motor planning and evaluation.
Figures

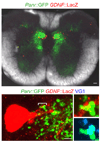

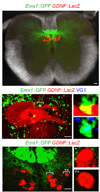
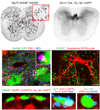
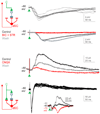
Comment in
-
Spinal convergence of motor and sensory pathways.Nat Neurosci. 2010 Oct;13(10):1160. doi: 10.1038/nn1010-1160. Nat Neurosci. 2010. PMID: 20877279 No abstract available.
References
-
- Lundberg A. Ascending Spinal Hindlimb Pathways in the Cat. Prog Brain Res. 1964;12:135–163. - PubMed
-
- Oscarsson O. Functional Organization of the Spino- and Cuneocerebellar Tracts. Physiol Rev. 1965;45:495–522. - PubMed
-
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 2001;81:539–568. - PubMed
-
- Matsushita M, Hosoya Y. Cells of origin of the spinocerebellar tract in the rat, studied with the method of retrograde transport of horseradish peroxidase. Brain Res. 1979;173:185–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

