Metabolic constituents of grapevine and grape-derived products
- PMID: 20835385
- PMCID: PMC2928446
- DOI: 10.1007/s11101-009-9158-0
Metabolic constituents of grapevine and grape-derived products
Abstract
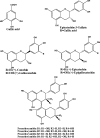
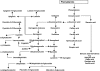
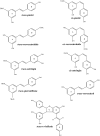
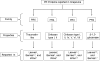
The numerous uses of the grapevine fruit, especially for wine and beverages, have made it one of the most important plants worldwide. The phytochemistry of grapevine is rich in a wide range of compounds. Many of them are renowned for their numerous medicinal uses. The production of grapevine metabolites is highly conditioned by many factors like environment or pathogen attack. Some grapevine phytoalexins have gained a great deal of attention due to their antimicrobial activities, being also involved in the induction of resistance in grapevine against those pathogens. Meanwhile grapevine biotechnology is still evolving, thanks to the technological advance of modern science, and biotechnologists are making huge efforts to produce grapevine cultivars of desired characteristics. In this paper, important metabolites from grapevine and grape derived products like wine will be reviewed with their health promoting effects and their role against certain stress factors in grapevine physiology.
Figures

References
-
- Adrian M, Jeandet P, Bessis R, Joubert JM. Induction of phytoalexin (resveratrol) synthesis in grapevine leaves treated with aluminum chloride (AlCl3) J Agric Food Chem. 1996;44:1979–1981.
-
- Adrian M, Jeandet P, Veneau J, Weston LA, Bessis R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J Chem Ecol. 1997;23:1689–1702.
-
- Adrian M, Jeandet P, Breuil AC, Levite D, Debord S, Bessis R. Assay of resveratrol and derivative stilbenes in wines by direct injection high performance liquid chromatography. Am J Enol Vitic. 2000;51:37–41.
-
- Ait-Barka E, Eullaffroy P, Clement C, Vernet G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004;22:608–614. - PubMed
-
- Alcalde-Eon C, Escribano-Bailon MT, Santos-Buelga C, Rivas Gonzalo JC. Changes in the detailed pigment composition of red wine maturity and ageing—a comprehensive study. Anal Chem Acta. 2006;563:238–254.
LinkOut - more resources
Full Text Sources
Other Literature Sources
