Arsenite stabilizes HIF-1α protein through p85α-mediated up-regulation of inducible Hsp70 protein expression
- PMID: 20835880
- PMCID: PMC3758129
- DOI: 10.1007/s00018-010-0459-7
Arsenite stabilizes HIF-1α protein through p85α-mediated up-regulation of inducible Hsp70 protein expression
Abstract
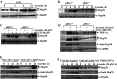
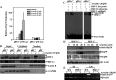
Hypoxia-inducible factor-1α (HIF-1α) has been reported to regulate over 100 gene expressions in response to hypoxia and other stress conditions. In the present study, we found that arsenite could induce HIF-1α protein accumulation in both mouse epidermal Cl41 cells and mouse embryonic fibroblasts (MEFs). Knockout of p85α, a regulatory subunit of PI-3K, in MEFs (p85α(-/-)) dramatically decreased the arsenite-induced HIF-1α accumulation, indicating that p85α is crucial for arsenite effects on the stabilization of HIF-1α protein. Our further studies suggest that arsenite could induce inducible Hsp70 expression, and transfection of inducible Hsp70 into p85α(-/-) MEFs could restore HIF-1α protein accumulation. Moreover, the results using EMSA and Supershift assays indicate that p85α is crucial for arsenite-induced activation of the heat-shock transcription factor 1 (HSF-1), which is responsible for transcription of inducible Hsp70. Taken together, p85α-mediated HIF-1α stabilization upon arsenite exposure is specifically through HSF-1 activation and subsequent up-regulation of the inducible Hsp70 expression.
Conflict of interest statement
There is no conflict interest for all authors listed.
Figures





Similar articles
-
PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation.J Biol Chem. 2004 Apr 2;279(14):13506-13. doi: 10.1074/jbc.M310164200. Epub 2004 Jan 15. J Biol Chem. 2004. PMID: 14726529
-
Differential transcriptional regulation of hypoxia-inducible factor-1α by arsenite under normoxia and hypoxia: involvement of Nrf2.J Mol Med (Berl). 2016 Oct;94(10):1153-1166. doi: 10.1007/s00109-016-1439-7. Epub 2016 Jun 10. J Mol Med (Berl). 2016. PMID: 27286880 Free PMC article.
-
DNA-dependent protein kinase is involved in heat shock protein-mediated accumulation of hypoxia-inducible factor-1alpha in hypoxic preconditioned HepG2 cells.FEBS J. 2008 Dec;275(23):5969-81. doi: 10.1111/j.1742-4658.2008.06725.x. FEBS J. 2008. PMID: 19021771
-
JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70.Cancer Res. 2010 Jan 15;70(2):813-23. doi: 10.1158/0008-5472.CAN-09-0448. Epub 2010 Jan 12. Cancer Res. 2010. PMID: 20068160 Free PMC article.
-
RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization.Cell Cycle. 2007 Mar 15;6(6):656-9. doi: 10.4161/cc.6.6.3981. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17361105 Review.
Cited by
-
GADD45α induction by nickel negatively regulates JNKs/p38 activation via promoting PP2Cα expression.PLoS One. 2013;8(3):e57185. doi: 10.1371/journal.pone.0057185. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23536762 Free PMC article.
-
The role of hypoxia-inducible factor 1 alpha (HIF-1α) modulation in heavy metal toxicity.Arch Toxicol. 2023 May;97(5):1299-1318. doi: 10.1007/s00204-023-03483-7. Epub 2023 Mar 18. Arch Toxicol. 2023. PMID: 36933023 Review.
-
NF-κB1 p50 promotes p53 protein translation through miR-190 downregulation of PHLPP1.Oncogene. 2014 Feb 20;33(8):996-1005. doi: 10.1038/onc.2013.8. Epub 2013 Feb 11. Oncogene. 2014. PMID: 23396362 Free PMC article.
-
A MALAT1/HIF-2α feedback loop contributes to arsenite carcinogenesis.Oncotarget. 2016 Feb 2;7(5):5769-87. doi: 10.18632/oncotarget.6806. Oncotarget. 2016. PMID: 26735578 Free PMC article.
-
Cyclooxygenase-2 (COX-2) mediates arsenite inhibition of UVB-induced cellular apoptosis in mouse epidermal Cl41 cells.Curr Cancer Drug Targets. 2012 Jul;12(6):607-16. doi: 10.2174/156800912801784802. Curr Cancer Drug Targets. 2012. PMID: 22463588 Free PMC article.
References
-
- Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. - DOI - PMC - PubMed
-
- Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha→hypoxia response element→VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000;60:6248–6252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

