Stable cyclic carbenes and related species beyond diaminocarbenes
- PMID: 20836099
- PMCID: PMC3130005
- DOI: 10.1002/anie.201000165
Stable cyclic carbenes and related species beyond diaminocarbenes
Abstract
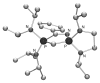
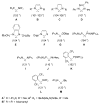
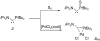
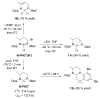
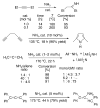
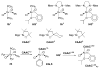
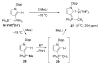
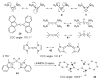
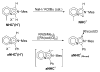
The success of homogeneous catalysis can be attributed largely to the development of a diverse range of ligand frameworks that have been used to tune the behavior of various systems. Spectacular results in this area have been achieved using cyclic diaminocarbenes (NHCs) as a result of their strong σ-donor properties. Although it is possible to cursorily tune the structure of NHCs, any diversity is still far from matching their phosphorus-based counterparts, which is one of the great strengths of the latter. A variety of stable acyclic carbenes are known, but they are either reluctant to bind metals or they give rise to fragile metal complexes. During the last five years, new types of stable cyclic carbenes, as well as related carbon-based ligands (which are not NHCs), and which feature even stronger σ-donor properties have been developed. Their synthesis and characterization as well as the stability, electronic properties, coordination behavior, and catalytic activity of the ensuing complexes are discussed, and comparisons with their NHC cousins are made.
Figures






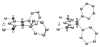









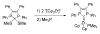


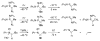


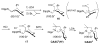





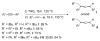



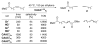

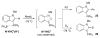
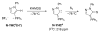

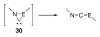


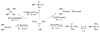

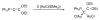
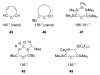











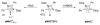

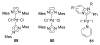





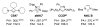

References
-
- Arduengo AJ, III, Harlow RL, Kline M. J Am Chem Soc. 1991;113:361–363.
-
- Igau A, Grützmacher H, Baceiredo A, Bertrand G. J Am Chem Soc. 1988;110:6463–6466.
- Igau A, Baceiredo A, Trinquier G, Bertrand G. Angew Chem. 1989;101:617–618.
- Angew Chem Int Ed Engl. 1989;28:621–622.
-
-
The first X-ray diffraction study of a (phosphino)silylcarbene was only reported in 2000: Kato T, Gornitzka H, Baceiredo A, Savin A, Bertrand G. J Am Chem Soc. 2000;122:998–999.
-
-
- Arduengo AJ, III, Harlow RL, Kline M. J Am Chem Soc. 1991;113:2801–2801.
-
- Bourissou D, Guerret O, Gabbaï FP, Bertrand G. Chem Rev. 2000;100:39–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

