Reactive oxygen species mediate oxidized low-density lipoprotein-induced inhibition of oct-4 expression and endothelial differentiation of bone marrow stem cells
- PMID: 20836655
- PMCID: PMC2971633
- DOI: 10.1089/ars.2010.3156
Reactive oxygen species mediate oxidized low-density lipoprotein-induced inhibition of oct-4 expression and endothelial differentiation of bone marrow stem cells
Abstract
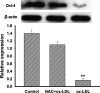
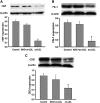
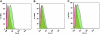
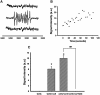
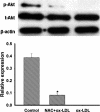
This study was to test the hypothesis that oxidized low-density lipoprotein (ox-LDL) modified the behavior of bone marrow stem cells, including proliferation, Oct-4 expression, and their endothelial differentiation through reactive oxygen species (ROS) formation in vitro. Rat bone marrow multipotent adult progenitor cells (MAPCs) were treated with ox-LDL with or without the antioxidant N-acetylcysteine (NAC). Ox-LDL generated a significant amount of ROS in the culture system as measured with electron paramagnetic resonance spectroscopy, and substantially inhibited the proliferation, Oct-4 expression, and endothelial differentiation of MAPCs. ROS production from ox-LDL in the culture system was completely prevented by NAC (1 mM). NAC treatment completely restored endothelial differentiation potential of MAPCs that was diminished by low-dose ox-LDL. NAC also significantly, but not completely, reversed the inhibitory effect of ox-LDL on proliferation and Oct-4 expression in MAPCs. NAC treatment only slightly restored Akt phosphorylation impaired by ox-LDL in the cells. ROS formation was important in the action of ox-LDL on MAPCs, including Oct-4 expression, proliferation, and endothelial differentiation. However, other mechanism(s) like Akt signaling and apoptosis might also play a critical role in mediating the effect of ox-LDL on these cells.
Figures
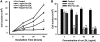



References
-
- Augé N. Nikolova-Karakashian M. Carpentier S. Parthasarathy S. Nègre-Salvayre A. Salvayre R. Merrill AH., Jr Levade T. Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem. 1999;274:21533–21538. - PubMed
-
- Bedel A. Nègre-Salvayre A. Heeneman S. Grazide MH. Thiers JC. Salvayre R. Maupas-Schwalm F. E-cadherin/beta-catenin/T-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ Res. 2008;103:694–701. - PubMed
-
- Breyer A. Estharabadi N. Oki M. Ulloa F. Nelson-Holte M. Lien L. Jiang Y. Multipotent adult progenitor cell isolation and culture procedures. Exp Hematol. 2006;34:1596–1601. - PubMed
-
- Chen JW. Mehta JL. Haider N. Zhang XJ. NArula J. Li DY. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

