Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase
- PMID: 20836852
- PMCID: PMC2949798
- DOI: 10.1186/1476-4598-9-239
Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase
Expression of concern in
-
Editorial expression of concern: Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase.Mol Cancer. 2025 Mar 31;24(1):103. doi: 10.1186/s12943-024-02185-7. Mol Cancer. 2025. PMID: 40165270 Free PMC article. No abstract available.
Abstract
Background: Ceramide is an important second messenger that has diverse cellular and biological effect. It is a specific and potent inducer of apoptosis and suppressor of cell growth. In leukemia, chemoresistance generally developed due to deregulated ceramide metabolism. In combinatorial treatment strategies of leukemia, few components have the capability to increases ceramide production. Manipulation in ceramide production by physiological and pharmacological modulators therefore will give additive effect in leukemia chemotherapy.
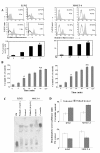
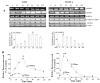
Results: Here, we show that Withanolide D (C4β-C5β,C6β-epoxy-1-oxo-,20β, dihydroxy-20S,22R-witha-2,24-dienolide; WithaD), a pure herbal compound isolated from Withania somnifera could effectively induces apoptosis in a dose and time dependant manner both in myeloid (K562) and lymphoid (MOLT-4) cells being nontoxic to normal lymphocytes and control proliferative cells. WithaD potentially augment ceramide production in these cells. Downstream of ceramide, WithaD acted on MKK group of proteins and significantly increased JNK and p38MAPK phosphorylation. Pharmacological inhibition of p38MAPK and JNK proves their cooperative action on WithaD-induced cell death. Dissecting the cause of ceramide production, we found activation of neutral sphingomyelinase and showed neutral-sphingomyelinase 2 (N-SMase 2) is a critical mediator of WithaD-induced apoptosis. Knockdown of N-SMase 2 by siRNA and inhibitor of N-SMase (GW4869) significantly reduced WithaD-induced ceramide generation and phosphorylation of MKK4 and MKK3/6, whereas phosphorylation of MKK7 was moderately regulated in leukemic cells. Also, both by silencing of N-SMase 2 and/or blocking by GW4869 protects these cells from WithaD-mediated death and suppressed apoptosis, whereas Fumonisin B1, an inhibitor of ceramide synthase, did not have any effect. Additionally, WithaD effectively induced apoptosis in freshly isolated lymphoblasts from patients and the potent cell killing activity was through JNK and p38MAPK activation.
Conclusion: Our results demonstrate that WithaD enhance the ceramide accumulation by activating N-SMase 2, modulate phosphorylation of the JNK and p38MAPK and induced apoptosis in both myeloid and lymphoid cells along with primary cells derived from leukemia patients. Taken together, this pure herbal compound (WithaD) may consider as a potential alternative tool with additive effects in conjunction with traditional chemotherapeutic treatment, thereby accelerate the process of conventional drug development.
Figures






Similar articles
-
Peptidoglycan induces cyclooxygenase-2 expression in macrophages by activating the neutral sphingomyelinase-ceramide pathway.J Biol Chem. 2009 Jul 31;284(31):20562-73. doi: 10.1074/jbc.M109.028084. Epub 2009 Jun 15. J Biol Chem. 2009. PMID: 19531467 Free PMC article.
-
Ceramide mediates Ox-LDL-induced human vascular smooth muscle cell calcification via p38 mitogen-activated protein kinase signaling.PLoS One. 2013 Dec 16;8(12):e82379. doi: 10.1371/journal.pone.0082379. eCollection 2013. PLoS One. 2013. PMID: 24358176 Free PMC article.
-
Stress-activated kinase pathway alteration is a frequent event in bladder cancer.Urol Oncol. 2012 Jul-Aug;30(4):415-20. doi: 10.1016/j.urolonc.2010.03.002. Epub 2011 Dec 11. Urol Oncol. 2012. PMID: 22154358
-
Stress-induced apoptosis and the sphingomyelin pathway.Biochem Pharmacol. 1997 Mar 7;53(5):615-21. doi: 10.1016/s0006-2952(96)00834-9. Biochem Pharmacol. 1997. PMID: 9113079 Review.
-
Ceramide in stress response.Adv Exp Med Biol. 2010;688:86-108. doi: 10.1007/978-1-4419-6741-1_6. Adv Exp Med Biol. 2010. PMID: 20919648 Free PMC article. Review.
Cited by
-
Neutral sphingomyelinase 2 modulates cytotoxic effects of protopanaxadiol on different human cancer cells.BMC Complement Altern Med. 2013 Jul 27;13:194. doi: 10.1186/1472-6882-13-194. BMC Complement Altern Med. 2013. PMID: 23889969 Free PMC article.
-
Withanolide D Enhances Radiosensitivity of Human Cancer Cells by Inhibiting DNA Damage Non-homologous End Joining Repair Pathway.Front Oncol. 2020 Jan 8;9:1468. doi: 10.3389/fonc.2019.01468. eCollection 2019. Front Oncol. 2020. PMID: 31970089 Free PMC article.
-
A new insight into identification of in silico analysis of natural compounds targeting GPR120.Netw Model Anal Health Inform Bioinform. 2018;7(1):8. doi: 10.1007/s13721-018-0166-0. Epub 2018 May 14. Netw Model Anal Health Inform Bioinform. 2018. PMID: 29780684 Free PMC article.
-
Ashwagandha attenuates TNF-α- and LPS-induced NF-κB activation and CCL2 and CCL5 gene expression in NRK-52E cells.BMC Complement Altern Med. 2015 Dec 15;15:434. doi: 10.1186/s12906-015-0958-z. BMC Complement Altern Med. 2015. PMID: 26667305 Free PMC article.
-
Withanolide D Exhibits Similar Cytostatic Effect in Drug-Resistant and Drug-Sensitive Multiple Myeloma Cells.Front Pharmacol. 2017 Sep 8;8:610. doi: 10.3389/fphar.2017.00610. eCollection 2017. Front Pharmacol. 2017. PMID: 28943850 Free PMC article.
References
-
- Smyth ML, Obeid LM, Hannun YA. Ceramide: a novel lipid mediator of apoptosis. Adv Pharmacol. 1997;41:133–154. full_text. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

