Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2
- PMID: 20837017
- PMCID: PMC2997180
- DOI: 10.1053/j.gastro.2010.09.002
Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2
Abstract
Background & aims: The tumor suppressor PTEN inhibits AKT2 signaling; both are aberrantly expressed in liver tumors. We investigated how PTEN and AKT2 regulate liver carcinogenesis. Loss of PTEN leads to spontaneous development of liver tumors from progenitor cells. We investigated how the loss of PTEN activates liver progenitor cells and induces tumorigenesis.
Methods: We studied mice with liver-specific disruptions in Pten and the combination of Pten and Akt2 to investigate mechanisms of liver carcinogenesis.
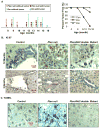
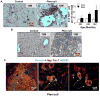
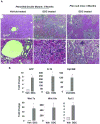
Results: PTEN loss leads to hepatic injury and establishes selective pressure for tumor-initiating cells (TICs), which proliferate to form mixed-lineage tumors. The Pten-null mice had increasing levels of hepatic injury before proliferation of hepatic progenitors. Attenuation of hepatic injury by deletion of Akt2 reduced progenitor cell proliferation and delayed tumor development. In Pten/Akt2-null mice given 3,5-diethoxycarbonyl-1,4 dihydrocollidine (DDC), we found that the primary effect of AKT2 loss was attenuation of hepatic injury and not inhibition of progenitor-cell proliferation in response to injury.
Conclusions: Liver carcinogenesis in Pten-null mice requires not only the transformation of TICs but selection pressure from hepatic injury and cell death, which activates TICs. Further research is required to elucidate the mechanism for hepatic injury and its relationship with TIC activation.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no conflicts of interest to disclose for all authors.
Figures




References
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews. 2009;9:265–273. - PubMed
-
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews. 2008;8:755–768. - PubMed
-
- Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem cell reviews. 2005;1:253–260. - PubMed
-
- Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Seminars in liver disease. 2003;23:385–396. - PubMed
-
- Dunsford HA, Karnasuta C, Hunt JM, Sell S. Different lineages of chemically induced hepatocellular carcinoma in rats defined by monoclonal antibodies. Cancer research. 1989;49:4894–4900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

