Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions
- PMID: 20837166
- PMCID: PMC3691868
- DOI: 10.1016/j.resp.2010.09.006
Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions
Abstract
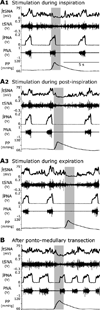
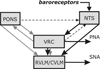
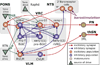
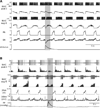
Sympathetic nerve activity (SNA) is modulated by respiratory activity which indicates the existence of direct interactions between the respiratory and sympathetic networks within the brainstem. Our experimental studies reveal that T(E) prolongation evoked by baroreceptor stimulation varies with respiratory phase and depends on the pons. We speculate that the sympathetic baroreceptor reflex, providing negative feedback from baroreceptors to the rostral ventrolateral medulla and SNA, has two pathways: one direct and independent of the respiratory-sympathetic interactions and the other operating via the respiratory pattern generator and is hence dependent on the respiratory modulation of SNA. Our experimental studies in the perfused in situ rat preparation and complementary computational modelling studies support the hypothesis that baroreceptor activation during expiration prolongs the T(E) via transient activation of post-inspiratory and inhibition of augmenting expiratory neurones of the Bötzinger Complex (BötC). We propose that these BötC neurones are also involved in the respiratory modulation of SNA, and contribute to the respiratory modulation of the sympathetic baroreceptor reflex.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Baekey DM, Dick TE, Paton JFR. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp. Physiol. 2008;93:803–816. - PubMed
-
- Barman SM, Gebber GL. Sympathetic nerve rhythm of brain stem origin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1980;239:R42–R47. - PubMed
-
- Bianchi AL, Denavitsaubie M, Champagnant J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol. Rev. 1995;75:1–45. - PubMed
-
- Bisset GS, 3rd, Gaum W, Kaplan S. The ice bag: a new technique for interruption of supraventricular tachycardia. J. Pediatr. 1980;97:593–595. - PubMed
-
- Brunner MJ, Sussman MS, Greene AS, Kallman CH, Shoukas AA. Carotid sinus baroreceptor reflex control of respiration. Circ. Res. 1982;51:624–636. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

